This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Q&A: Building a future for testing medical products using non-animal models
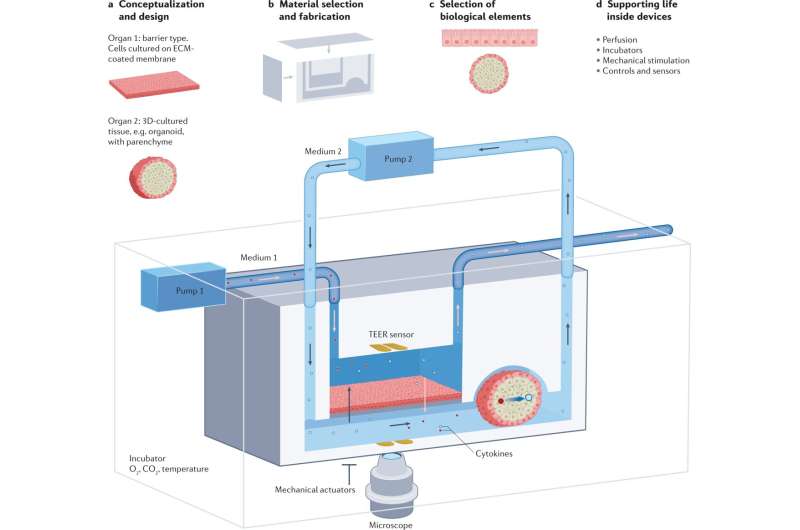
For a long time, we've relied on animal testing to advance human health. It's been considered necessary to develop new and effective medical treatments that save human lives. But what if we could reduce or even eliminate the need for animal testing altogether? What if we could turn to innovative, ethical alternatives that are not only more effective but also more humane?
We recently released a report titled "Non-animal models: A strategy maturing Australia's medical product development capabilities." The report assesses the potential of non-animal models to complement or replace traditional animal models over the next 15 years.
Greg Williams is our CSIRO Futures' Health and Biosecurity lead and is one of the authors of the report. We sat down with Greg to discuss the report and explore the steps required to accelerate the demonstration, scaling, and commercial success of non-animal models in Australia.
What are non-animal models and how can they support medical product development?
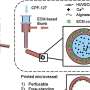
Traditional animal models, like mice and ferrets, are widely used to test the safety of new medical products. However, non-animal models that use human cells, tissues and data, have the potential to offer the same services. They come in various forms, from advanced computer simulations to tissue samples from patients, and even revolutionary three-dimensional (3D) models like organs-on-chips.
Non-animal models could benefit research and testing right across the medical product development process. This ranges from fundamental research (aimed at improving general understanding) through to clinical trials (testing a specific medical product).
By using human-derived components, non-animal models have the potential to predict human responses more accurately than animal models. This could speed up the identification of effective drug candidates allowing resources to be redirected towards more promising options. These models also better align with ethical considerations by offering a pathway to reducing animal use.
How are non-animal models being used, and what opportunities lie ahead?
Some alternatives to animal testing are already being used in medical research. For instance, scientists use two-dimensional (2D) cell cultures, where they grow cells in a flat layer, to identify potential drug targets during the early stages of drug development. This method helps researchers pinpoint specific proteins or genes that a new drug could target to treat a disease.
These 2D models are especially good for high-throughput screening (using automated screening to test large numbers of samples). This technique allows scientists to test a large number of compounds and conditions quickly and efficiently. This is much more efficient than using animal models and accelerates drug development.
Animal models are still used for most other steps in medical product development. However, computer simulations and more complex 3D non-animal models are used to validate animal model findings. They can also offer complementary insights.
The future holds numerous opportunities. Our report identified four key areas where Australia's strengths align with global needs. These are:
- Drug candidate screening: enabling high-throughput screening for potential drug candidates.
- Toxicology testing: ensuring the safety of new medical products.
- Informing patient selection for clinical trials: using patient cells to identify the most suitable treatments.
- Manufacturing model components: such as stem cells and hydrogels.
There is also substantial economic potential. Our analysis suggests by 2040, the combined annual revenue from organoids and organ-on-chip technologies could reach an estimated $1.6 billion, creating more than 5,000 new jobs in Australia.
How does Australia compare globally?
Australia has world-class researchers and institutions that are generating non-animal models. We analyzed Australia's output data across research, clinical and industry sectors to identify areas of competitive strength. This analysis indicated Australia was globally competitive for 3D cardiovascular models, 3D nervous system models, and 2D respiratory models. While other model types were identified as research strengths, they have not yet been applied to clinical or industry applications.
It is widely acknowledged the United States (US) and European Union (EU) are leading the world when it comes to non-animal models. In these regions, non-animal models are more formally acknowledged as critical technologies, supported by dedicated funding programs, governing bodies and policy and legislative changes that encourage their use.
How can Australia pursue these opportunities?
There are two broad categories of activity needed in Australia. The first is about continuing to improve the quality of non-animal models. This is primarily the responsibility of the research community. This includes generating as much evidence as possible that these models are on par with or superior to their animal model equivalents. We also need to drive the costs down further so that the transition towards non-animal models has a strong economic basis.
The second category relates to strengthening the supporting ecosystem around this technical capability. This is primarily the responsibility of governments. However, it will require strong collaboration with industry and research. These efforts could include policy and regulation updates that encourage non-animal model investments. We need to establish national data collection standards for animal use. We also need national networks for the coordination and promotion of local activities, skills development and investments in updated and open access infrastructure.
More information: Non-animal models: A strategy maturing Australia's medical product development capabilities. www.csiro.au/en/work-with-us/s … ty/Non-animal-models