October 2, 2023 feature
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New tests of a recently approved RSV vaccine show potent antibody response to current and past variants
New tests of a recently approved vaccine for respiratory syncytial virus—RSV—show the shot remains effective against a range of variants producing potent antibody responses against current and past strains, and may even bode well against future viral offshoots.
The new research, led by scientists in Belgium, involved small and large animals as well as antibody samples from older human adults. The positive antibody response against the virus was particularly evident when the vaccine was combined with an adjuvant, which is an additional ingredient to boost the immune response.
The new research arrives as seasonal viruses begin their annual circulation throughout the Northern Hemisphere—and public health officials wait with baited breath to gauge whether a "tripledemic" could mark the 2023–2024 season. COVID cases have already gotten a jump on the season in many countries, including the United States and the United Kingdom. Whether RSV and influenza will be more or less aggressive has yet to be determined.
"RSV is a major cause of lower respiratory tract diseases in young children and older adults," asserted Lionel Sacconnay, lead author of the new research. "Two antigenically distinct RSV subtypes, RSV-A and –B, co-circulate worldwide, with each subtype being composed of multiple genotypes. Several vaccine candidates were recently shown to be efficacious in protecting older adults against RSV-associated lower respiratory tract diseases in clinical trials."
To date, only one of those vaccines—RSVPreF3—has been approved, and it is the one under study for long-term effectiveness by Sacconnay and his team. The difference between RSVPreF3 and the one in Sacconnay's experiments, is the one under study is dubbed RSVPreF3-AS01, which means that it has the AS01 adjuvant added to the formulation. Vaccine adjuvants are added as an extra kicker to jump-start the immune response. The practice of adding adjuvants to vaccines is more common in Europe than in the United States.
AS01 is a liposome-based vaccine adjuvant system containing two immuno-stimulants: 3-O-desacyl-4'-monophosphoryl lipid A and the saponin QS-21. Saponins are both water and fat soluble. One well known saponin is the compound glycyrrhizin, commonly known as the flavor of licorice.
"The vaccine has been very recently approved by the U.S. Food and Drug Administration and the European Medicines Agency for the prevention of lower respiratory tract diseases caused by RSV in individuals 60 years of age and older," noted Sacconnay a scientist with GSK, GlaxoSmithKline, the maker of the vaccine.
The take home message from the team's new research: Experiments indicate the vaccine could offer enduring protection against contemporary RSV strains and future variants.
Writing in Science Translational Medicine, Sacconnay and colleagues analyzed the RSV vaccine (sold in the U.S. under the brand name Arexvy) to determine whether neutralizing antibodies prompted by it remained robust and durable. The new Belgium-based experiments analyzed the vaccine along with the AS01 adjuvant.
The COVID-19 pandemic has widely demonstrated that vaccine-induced immunity can become less effective over time with the vaccine sometimes losing potency against new variants as the virus mutates. Although RSV doesn't seem to mutate as quickly as SARS-CoV-2 or the annual influenza viruses, studies are still needed to determine whether the RSV vaccine will remain effective with emerging viral mutations.
The team already knows where those mutations are most likely to occur. Scientists have pinpointed the molecular location of eight amino acid residue sites in the viral structure where variation may arise, affecting the viral fusion protein in its pre-fusion conformation.
All viruses mutate and change their molecular character, some becoming more virulent, others becoming less so and milder. RSV is no exception to the mutation rule. Its fusion protein, which it uses to initiate infection with a human cell, is more highly conserved compared with other respiratory viruses. That doesn't mean there will be no variation in the fusion protein over time, but that changes are likely to be less frequent. With that said, scientists underscore that there is still enough variation in circulating RSV variants to affect the vaccine.
"The RSV envelope contains two major glycoproteins, the fusion protein, F, and the cell attachment G protein," Scconnay explained. "F is responsible for viral cell membrane fusion allowing RSV entry into the host cell."
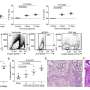
To determine if the new vaccine will help prompt neutralizing antibodies regardless of the variant, the GSK team turned to a vast number of viral variants to further the experiments. Using a large dataset of RSV sequences gathered from 1990 to 2022, the scientists first characterized the most noticeable changes in the virus's major antigenic sites over time.
Then, they selected a panel of six RSV strains with different combinations of these major variations. To their pleasant surprise, they found that antibodies induced by the RSV vaccine neutralized all the variants in culture. The experiments did not end there.
The Belgium-based team then conducted additional tests and found the vaccine elicited broad antibody responses in mice and in cows effectively neutralizing the six selected RSV strains, especially when combined with the vaccine adjuvant AS01.
A study of serum samples from vaccinated older adults also showed that, with the AS01 adjuvant added, the vaccine produced a strong antibody response against the RSV strains, suggesting that the adjuvant could help maintain the vaccine's efficacy.
"We expect RSVPreF3-AS01 to protect older adults against lower respiratory tract disease not only caused by any contemporary RSV strain, but also by those that may emerge in the future," Sacconnay wrote, noting particular effectiveness of the experimental vaccine formulation with the AS01 adjuvant.
More information: Lionel Sacconnay et al, The RSVPreF3-AS01 vaccine elicits broad neutralization of contemporary and antigenically distant respiratory syncytial virus strains, Science Translational Medicine (2023). DOI: 10.1126/scitranslmed.adg6050
© 2023 Science X Network