This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Tirzepatide weight-loss study shows promising results
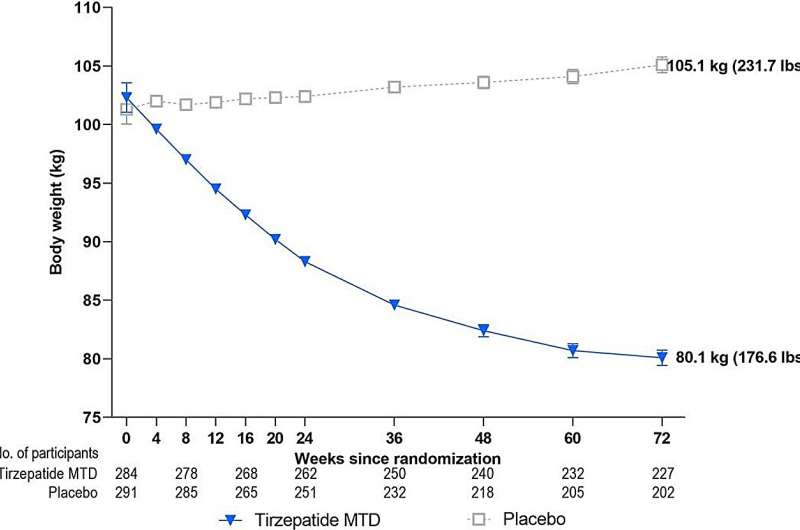
After 12 initial weeks of weight loss with intensive lifestyle intervention alone, participants in the SURMOUNT-3 study who were randomly assigned to tirzepatide for 72 weeks achieved a total mean reduction in baseline body weight of 24.3% at week 84.
Results of the study, conducted by Eli Lilly & Company, were presented during the 41st Annual Meeting of The Obesity Society (TOS) at ObesityWeek 2023 scheduled for Oct. 14–17, in Dallas, Texas. The study was published in the journal Nature Medicine to coincide with the presentation at the conference.
"These are extraordinary findings, which show that participants—who had already lost 6.9% of their baseline body weight with traditional diet and activity counseling—lost an additional 18.4% of body weight when administered tirzepatide, compared with a gain of 2.5% in participants assigned to placebo," said TOS Past President Thomas Wadden, Ph.D., FTOS, professor of psychology in psychiatry, Perelman School of Medicine, University of Pennsylvania, Philadelphia. Wadden is the lead author of the paper.
"The additional weight loss produced further improvements, compared with placebo, in multiple measures of health, including waist circumference, blood pressure, cholesterol and triglycerides, blood sugar and physical functioning."
Ariana Chao, Ph.D., MSN, RN, FNP-BC, faculty associate, Johns Hopkins University School of Nursing, Baltimore, Md, and second author of the paper, presented the study and discussed its efficacy findings at ObesityWeek.
"From the start of the intensive lifestyle intervention, participants treated with tirzepatide had an average body weight loss of 64 pounds. Health care providers have long sought strategies to help patients with obesity achieve losses of this magnitude, which can provide benefits to patients' health and quality of life," said Chao.
Wadden added "patients who received lifestyle intervention and tirzepatide achieved a mean weight loss consistent with that produced by sleeve gastrectomy, a widely used procedure in metabolic and bariatric surgery. Tirzepatide could offer a safe and highly effective alternative to surgery for some persons with severe obesity."
SURMOUNT-3 was a multi-center, randomized, double-blind, parallel, placebo-controlled trial that compared the efficacy and safety of tirzepatide to placebo for 72 weeks, after a 12-week intensive lifestyle intervention lead-in period in adults with obesity or overweight with weight-related comorbidities, excluding type 2 diabetes.
Tirzepatide is a once-weekly glucose-dependent insulinotropic polypeptide (GIP) receptor and glucagon-like peptide-1 (GLP-1) receptor agonist, combined in a single molecule. The drug activates the body's receptors for GIP and GLP-1, which are natural incretin hormones.
Both GIP and GLP-1 receptors are found in areas of the human brain important for appetite regulation. Tirzepatide has been shown to decrease food intake by increasing satiety and decreasing hunger and reward-based eating.
Multiple presentations of the findings discussed the study's background and rationale; the study design and participants' baseline characteristics; and the efficacy, safety and clinical results.
More information: Thomas A. Wadden et al, Tirzepatide after intensive lifestyle intervention in adults with overweight or obesity: the SURMOUNT-3 phase 3 trial, Nature Medicine (2023). DOI: 10.1038/s41591-023-02597-w