This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers unravel cross-species pain-preferential neural pathway
Researchers have uncovered a neural pathway preferentially involved in pain perception across species. The study was led by Profs. Tu Yiheng and Hu Li from the Institute of Psychology of the Chinese Academy of Sciences and was published in Nature Human Behaviour on Oct. 9.
Pain serves as a crucial signal to the human body, alerting us to potential harm or damage. For decades, scientists have debated whether there is any pain-preferential neural pattern in the human brain that distinguishes pain from other sensory modalities.
Traditionally, different sensations, including pain, were thought to be processed in separate unisensory areas, which then converge in higher order multisensory regions. However, recent evidence suggests that more integrated, multisensory processing occurs even in areas previously considered to be unisensory.
In this study, the researchers applied state-of-the-art analytical techniques to human neuroimaging (i.e., fMRI and EEG) and rat electrophysiology datasets to identify distinctive neural signatures associated with pain perception across species.
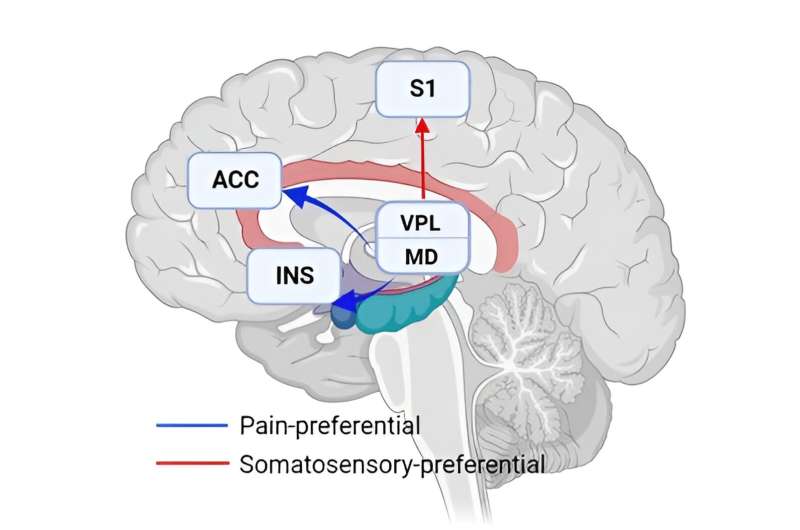
Through multivariate pattern analysis, the researchers revealed the central role of the medial-dorsal (MD) thalamic nucleus in pain perception. Painful stimuli elicited a more pronounced activation of this nucleus, and its rich connectivity with the dorsal anterior cingulate cortex (ACC) and insula also preferentially responded to pain.
Previous studies have found that most so-called "pain-evoked activities" are responsive to stimulus salience, rather than pain itself. Crucially, the preferential engagement of MD persisted even after controlling for stimulus salience, as shown by the MD nucleus's enhanced response to pain compared to equally salient electro-tactile stimuli.
Using an advanced fMRI-EEG fusion technique, the researchers further identified a significant time window, from 89 ms to 295 ms after the application of painful stimuli, during which the MD nucleus exhibited a preferential response to pain. This temporal framing provides unprecedented insight into the neural dynamics of pain perception, adding a new dimension to our understanding of how pain signals are processed in the brain.
To provide further evidence of the pain-preferential MD-ACC/insula pathway, the study was also extended to a rat model in order to validate the neural dynamics observed in humans. Consistent with the human study, painful stimuli preferentially activated MD neurons and MD-ACC connectivity in the rat model, which not only confirms the observations in humans, but also underscores a conserved pathway in pain perception across species.
This study sheds light on the complex neural dynamics underlying pain perception. By delineating this pain pathway, it expands our understanding of pain perception and opens up potential avenues for targeted intervention in pain management.
More information: Yiheng Tu et al, Pain-preferential thalamocortical neural dynamics across species, Nature Human Behaviour (2023). DOI: 10.1038/s41562-023-01714-6