This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Lung cancer researchers identify specific genetic change that predicts whether patients can respond to targeted therapy
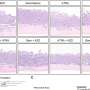
![(A) Overview of the study cohorts. (B) Kaplan-Meier curve showing progression-free survival of patients with 8p11-amplified SQLC treated with the FGFR inhibitors BGJ398 or GW786034 (TUM006). FGFR1 amplification was determined by FISH. Asterisk indicates that treatment was stopped because of toxicity. (C) Tumor volume change for patients with FGFR1-amplified SQLC treated with BGJ398 (Response Evaluation Criteria in Solid Tumors [RECIST] criteria). Tumor progression (red) and durable response (blue) following FGFR inhibition. TUM003 and TUM007 died during treatment with no sign of response. One patient (TUM006) was treated off-label (asterisk indicates that no RECIST data are available). Tumor shrinkage was estimated on CT scans. (D and E) CT scans of patient TUM005 without a response and patient TUM004 with a durable response. Credit: Journal of Clinical Investigation (2023). DOI: 10.1172/JCI170217 Another building block for personalized medicine for lung cancer is discovered](https://scx1.b-cdn.net/csz/news/800a/2023/another-building-block.jpg)
Squamous cell lung cancer is a lung cancer subtype that is particularly difficult to treat. A new study now has revealed a novel genetic alteration that occurs in some cases in this type of tumor and that may expose a weakness of the tumor for therapeutic intervention.
The University of Cologne researchers led by Professor Roman Thomas, director of the Department of Translational Genomics, was able to show that a certain genetic change occurs during tumor formation and that a previously unknown oncogene is produced. Oncogenes are genes that promote the growth of tumors. In some cases, they can be inhibited by targeted drug treatments.
This approach is often accompanied by a higher success rate and lower side effects compared to conventional chemotherapy. The scientists' discovery could therefore be a first step toward a more successful therapy of this particular type of cancer.
The study was published in the Journal of Clinical Investigation under the title "Somatic rearrangements causing oncogenic ectodomain deletions of FGFR1 in squamous cell lung cancer." A Commentary on this research by Netta Mäkinen and Matthew Meyerson was published in the same journal.
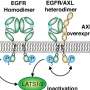
The new discovery concerns a genetic modification that leads to the removal of the "extracellular domain" of the FGFR1 protein (fibroblast growth factor receptor). This domain plays a crucial role in the activation and regulation of the FGFR1 protein. FGFR1 can be found in the cell membrane that separates the cell from its environment. Thus, the protein has both points of contact with the cell interior and with the environment.
The study shows that the loss of the extracellular domain, i.e., the part that protrudes outwards, leads to sustained growth signals in the tumor cells. The receptor no longer receives stop signals and the tumor continues to grow. One positive aspect is that FGFR inhibitors are already being used in clinical practice, for example for the treatment of bladder cancer. Such inhibitors are drugs that specifically bind to proteins and deactivate them.
"Hopefully, the new results will enable a new therapy option for a specific group of patients with squamous cell carcinoma of the lungs," said Dr. Florian Malchers, first author of the current study.
Thirteen years ago, scientists in Professor Thomas' laboratory described the amplification of FGFR1 in squamous cell carcinoma of the lung for the first time. Amplification is the multiple occurrence of gene copies in a tumor. Unfortunately, a first clinical study showed that the mere presence of amplification as a selection criterion for personalized FGFR therapy in patients is not yet sufficient.
Only 11% of patients benefited from treatment with an FGFR inhibitor. In this group of patients, the team has now been able to demonstrate a link between the genetic modification of the FGFR1 protein, the removal of the extracellular domain, and a significant decrease in tumor volume. These findings could therefore offer a new avenue of treatment for this group of patients.
More information: Florian Malchers et al, Somatic rearrangements causing oncogenic ectodomain deletions of FGFR1 in squamous cell lung cancer, Journal of Clinical Investigation (2023). DOI: 10.1172/JCI170217
Netta Mäkinen et al, Genomic insights into the mechanisms of FGFR1 dependency in squamous cell lung cancer, Journal of Clinical Investigation (2023). DOI: 10.1172/JCI174171