This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Miniature colons with immune components aid the study of intestinal diseases
A team at the Medical University of South Carolina and Cincinnati Children's has developed a sophisticated model for studying the diseased colon that could lead to the development of personalized treatments for colon-related diseases, such as cancer and inflammatory bowel disease (IBD). The researchers report their findings in Cell Stem Cell.
MUSC Hollings Cancer Center researcher Jorge Munera, Ph.D., collaborated with James Wells, Ph.D., and Daniel Kechele, Ph.D., both of Cincinnati Children's, to grow miniature human colons complete with an immune system in the lab. This model improves upon existing organoids, or mini-organs, which have no natural connection to immune components. These novel colon organoids more closely resemble the human colon in both healthy and diseased states.
"We think that this new model is significant because most gastrointestinal diseases involve the immune system and inflammation," said Munera, an assistant professor in the Department of Regenerative Medicine and Cell Biology at MUSC.
Until recently, methods for studying colon diseases, such as colon cancer and IBD, have been limited to cells and animal models. Cells are often derived from cancerous tumors, limiting their applicability to the study of non-cancer diseases. Animal models have their own set of limitations: Treatments that are promising in animals do not always provide the same benefit to humans.
A happy medium between cells and animals is provided by organoids, three-dimensional groups of cells that mimic organ functions. They are significantly more complex than traditional human cell cultures, but they still lack some features of complete human organs, and unlike an animal model, organoids are not connected to an entire body system.
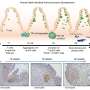
Munera, Wells, and their team have found a way to overcome one of the significant remaining limitations of colon organoids by inducing these next-generation organoids to develop early-stage immune cell types that naturally reside within colon tissue.
This study builds upon similar work published in Nature Biotechnology, which was led by Michael Helmrath, M.D., at Cincinnati Children's and co-authored by Wells and colleagues.
"Importantly, these immune cells are nearly identical to those found in the human body, where they are able to detect disease-causing bacteria and remove them," Wells said. "This is an important step for research aimed at identifying future therapies for IBD and other diseases impacting the gastrointestinal tract."
The research team generated colon organoids using stem cells obtained from patient blood samples. Stem cells are the uniform ancestor cells in the body that can develop into specific cell types required by the body. Under the right conditions, these stem cells can communicate with each other to form a colon organoid. The cells' communication also allows them to self-organize into layers similar to natural tissue organization in the body.
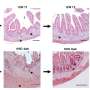
"They contain not only the lining of the colon but also the supporting cells and even some immune cells that grow along with the rest of this tissue," Munera explained.
The immune cells in these novel colon organoids are part of the innate immune system and act as the body's "first responders" to inflammation.
"We have made a more complete human organoid system that we can use to model inflammation in the colon," said Munera.
With more development, Munera thinks the novel organoid model could help to personalize treatments for diseases of the colon. In the future, these organoids could be generated from the blood of a patient with very early-onset IBD, for example, and used to test whether a treatment would work before administering it.
More information: Jorge O. Múnera et al, Development of functional resident macrophages in human pluripotent stem cell-derived colonic organoids and human fetal colon, Cell Stem Cell (2023). DOI: 10.1016/j.stem.2023.10.002