This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Semaglutide reduces cardiovascular events by 20% in overweight or obese adults who don't have diabetes: Trial
Findings from a multi-center, international clinical trial reported by a Cleveland Clinic physician show that semaglutide reduced cardiovascular events by 20% in adults with overweight or obesity and established cardiovascular disease who do not have diabetes.
Semaglutide is primarily prescribed for adults with type 2 diabetes but is also approved for chronic weight management in adults with obesity or overweight and have at least one other health issue. In the trial, patients treated with semaglutide lost an average of 9.4% of their body weight and experienced improvements in other risk factors for cardiovascular disease.
Results from the "SELECT—Semaglutide and Cardiovascular Outcomes in Patients with Overweight or Obesity Who Do Not Have Diabetes" trial were presented during a late-breaking science session at the American Heart Association's Scientific Sessions 2023 and simultaneously published in the New England Journal of Medicine.
In the trial, for patients with preexisting cardiovascular disease and overweight or obesity but without diabetes, weekly injections of semaglutide at a dose of 2.4 mg was superior to placebo in reducing the risk of death from cardiovascular causes, nonfatal heart attack, or nonfatal stroke over an average follow-up of 40 months.
"It is known that overweight and obesity increase a person's risk of cardiovascular events. Yet while reducing cardiovascular disease by treating high cholesterol, high blood pressure, and diabetes is standard practice, the concept of treating obesity to reduce cardiovascular complications has been hampered by the lack of evidence that lifestyle or pharmacologic interventions for overweight or obesity improve cardiovascular outcomes," said Michael Lincoff, M.D., SELECT's lead author and vice chair for research in Cleveland Clinic's Department of Cardiovascular Medicine.
"This marks the first pharmacologic intervention for overweight or obesity that's been shown in a rigorous fashion to reduce the risk of cardiovascular events."
More than half the world population is projected to have overweight or obesity by the year 2035. High body-mass index (BMI) is estimated to have accounted for 4 million deaths globally in 2015, more than two thirds of which were caused by cardiovascular diseases.
Semaglutide, a GLP-1 receptor agonist medication initially approved and most frequently prescribed for adults with type 2 diabetes, was also FDA-approved in 2021 for chronic weight management in adults with obesity or overweight with at least one weight-related comorbidity.
While the weight loss effects of semaglutide appear to occur primarily through appetite suppression, this drug has other actions which may reduce cardiovascular risk, including improvements in glucose levels, decreases in blood pressure and cholesterol levels and reductions in inflammation, and beneficial effects on heart muscle and blood vessels.
In the SELECT trial, which ran from October 2018 through June 2023, researchers enrolled patients 45 years of age or older who had pre-existingcardiovascular disease and a body mass index of 27 or greater but no history of diabetes. Over 17,000 patients in 41 countries who had previously experienced a heart attack, stroke and/or had peripheral artery disease were enrolled and followed for an average of 40 months after being randomly assigned to receive once weekly injections of semaglutide 2.4 mg or placebo.
In addition to taking either semaglutide or placebo for the trial, all participants also received standard-of-care treatment for cardiovascular disease, such as cholesterol-modifying medications, antiplatelet therapies, beta blockers or other treatments.
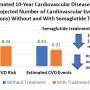
Death from a cardiovascular event, nonfatal myocardial infarction (heart attack), or nonfatal stroke occurred during the trial in 6.5% of patients who were treated with semaglutide versus 8.0% of patients who received placebo—a 20% reduction in relative risk by semaglutide. Risk reductions were similar in men and women and across different ethnicities, patient ages and baseline levels of bodyweight.
There were no unexpected safety issues with semaglutide in this trial. More patients discontinued semaglutide (16.6%) than placebo (8.2%), due primarily to gastrointestinal symptoms including nausea and diarrhea. These gastrointestinal symptoms are not uncommon with this class of medications, particularly when the drug is started, or the dose is increased.
There was a slightly higher rate of gallbladder disorders in the semaglutide vs. placebo group (2.8% vs. 2.3%, respectively), which has also been previously reported in other studies with GLP-1 agents. Importantly, semaglutide was not associated with higher risks for severe gastrointestinal disorders, pancreatitis, psychiatric disorders or kidney injury.
"There's growing recognition that obesity and overweight are really metabolic diseases, and yet, effective therapies have been quite limited," said Dr. Lincoff. "This study of semaglutide demonstrates the effectiveness of a new pathway to reduce the excess risk associated with obesity of important and potentially deadly cardiovascular complications."
One limitation of the trial is that only patients with pre-existing cardiovascular disease were included. The effects of semaglutide on primary prevention of cardiovascular events in persons with overweight or obesity, but without previous cardiovascular disease, were not studied.
More information: A. Michael Lincoff et al, Semaglutide and Cardiovascular Outcomes in Obesity without Diabetes, New England Journal of Medicine (2023). DOI: 10.1056/NEJMoa2307563