This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Sequencing unveils 14% triplicated α-globin genes among thalassemia carriers in Southern China
500,000 children are born with hemoglobinopathies annually, and 80% of them are born in developing countries. According to the Global Prevention and Control of Hemoglobinopathies report, carrier screening could potentially be provided to 113 million pregnant women every year.
Among hereditary hemoglobinopathies, thalassemia is the most prevalent, occurring in 4.4 out of every 10,000 live births. It is prevalent in Mediterranean coastal areas, Africa, the Middle East, Southeast Asia, and southern China.
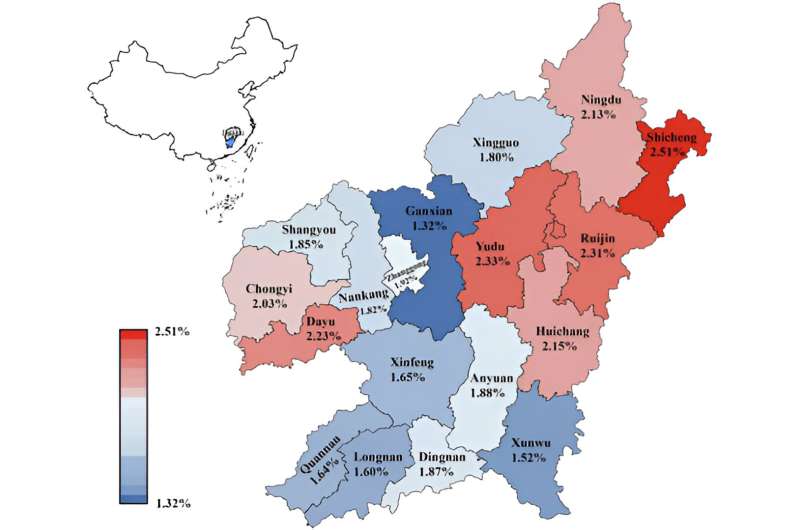
A new study led by Xinxing Xie, Jinhui Gan, Zezhang Liu, Wenqian Zhang (BGI Genomics Senior Manager Clinical Research) and recently published in Frontiers in Genetics has uncovered the prevalence and genetic intricacies of triplicated α-globin genes for 73,967 individuals in Ganzhou, a city located in southern China.
This extensive investigation sought to understand the prevalence, distribution, and clinical characteristics of individuals carrying triplicated α-globin genes, particularly those co-inherited with heterozygous β-thalassemia.
α-globin gene triplication is a rare genetic variant that arises from unequal genetic crossover events during meiosis, leading to an overproduction of α-globin proteins.
Typically, individuals with normal β-globin genes and triplicated α-globin genes do not manifest clinical symptoms or exhibit significant alterations in hematological parameters.
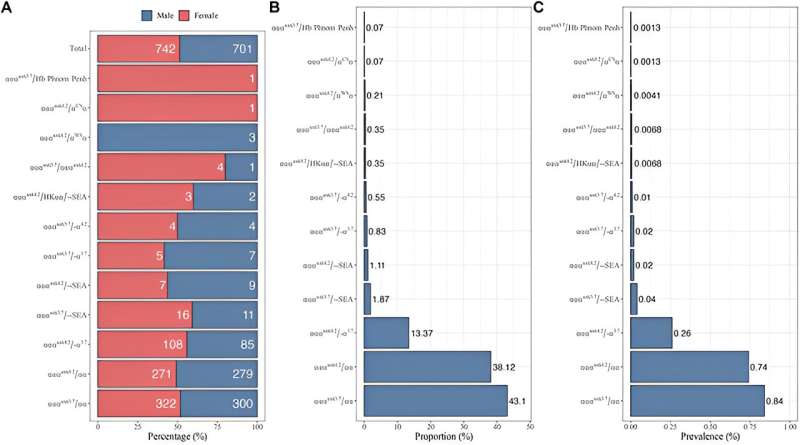
73,000 individuals from Ganzhou voluntarily underwent comprehensive genetic testing between 2021 and 2022, highlighting a carrier rate for α-globin gene triplication.
The most prevalent mutation identified was αααanti3.7/αα (constituting 43.10% of cases), followed closely by αααanti4.2/αα (at 38.12%).
This study made an intriguing discovery by identifying 42 individuals carrying both α-globin gene triplication and heterozygous β-thalassemia.
Surprisingly, these individuals did not display significant differences in hematological parameters such as Mean Corpuscular Volume (MCV) and Mean Corpuscular Hemoglobin (MCH) compared to individuals with heterozygous β-thalassemia and normal α-globin genes.
The study's follow-up assessments of eight subjects with both α-globin gene triplication and heterozygous β-thalassemia revealed a wide spectrum of clinical outcomes.
Notably, different degrees of anemia were observed, and even among individuals sharing identical genotypes, the severity of anemia varied.
A striking observation was the apparent random distribution of α-globin gene triplication across Ganzhou's counties, contrasting the typical geographic patterns associated with thalassemia distribution in China.
Key points to highlight:
1: The research findings challenge the previously held understanding of how α-globin gene triplication interacts with heterozygous β-thalassemia, highlighting a more intricate relationship.
2: Besides contributing valuable insights into Ganzhou's genetic landscape, these data expand our comprehension of thalassemia within a larger genetic context.
3: These findings underscore the importance of careful clinical assessment in such a scenario and open new pathways for exploring genetic variations within the realm of thalassemia.
Research results suggest that α-globin gene triplication should not be considered a high-risk factor in prenatal diagnosis or genetic counseling, and should not receive extensive clinical attention in healthy individuals. However, in heterozygous β-thalassemia carriers with corresponding symptoms, attention should be directed towards its potential involvement.
BGI Genomics Team's in-depth research represents a significant leap in our understanding of α-globin gene triplication, shedding light on its diverse clinical manifestations when co-inherited with heterozygous β-thalassemia. This study enriches our genetic knowledge by offering essential data for genetic counseling and informing future investigations into atypical thalassemia cases. It enhances clinical practices, serving as a cornerstone for further research into the complexities of genetic interactions and their impact on human health.
More information: Xinxing Xie et al, Prevalence and genetic analysis of triplicated α-globin gene in Ganzhou region using high-throughput sequencing, Frontiers in Genetics (2023). DOI: 10.3389/fgene.2023.1267892