This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Treating anemia with gene scissors
ETH Zurich molecular biologist Mandy Boontanrart is researching gene therapies that could be used to cure two of the most common types of inherited anemia. She has now developed a promising approach for so-called beta-hemoglobinopathies. The paper is published in the journal eLife.
Many hereditary diseases have largely been considered to be incurable. The intervention in the genome is too unpredictable and complex and the results of this manipulation too uncertain. This is because these diseases often involve not just one but several genes, which can be located on different chromosomes.
But with the phenomenal uptake of the CRISPR-Cas9 gene scissors, the rulebook has been extensively rewritten. In just the past few years, targeted manipulation of individual genes or even entire building blocks of DNA has come a very long way. The results of these collaborative efforts is that curing hereditary diseases in humans is now within reach.
Using gene scissors against beta-hemoglobinopathies
Among those looking to tackle a hereditary disease with CRISPR-Cas9 technology is molecular biologist Mandy Boontanrart from the group led by ETH Professor Jacob Corn. She recently worked on a study that may prove groundbreaking in the treatment of hereditary beta hemoglobinopathies. This term covers two types of anemia: beta thalassemia and sickle cell anemia, which are among the most common hereditary conditions in the world.
Beta hemoglobinopathies are caused by mutations of the HBB gene. This gene contains the blueprint for a protein chain called beta globin, a component of hemoglobin. Present in vast quantities in red blood cells, hemoglobin is what gives blood its color and is responsible for transporting oxygen throughout the body. Hemoglobin in adults is made up of two alpha globins and two beta globins. Present in lesser amounts, hemoglobin can be made up of two alpha globins and two delta globins. The delta globins work exactly like beta globins but are produced naturally in red blood cells only in tiny amounts.
If the HBB gene has a mutation that causes beta globin production to malfunction, there will be a shortage of functional hemoglobin. Typically, this can result in red blood cells dying off prematurely, leading to anemia. All the body's organs then suffer from a chronic lack of oxygen.
If the mutation is limited to just one copy of the HBB gene, carriers can lead a relatively normal life. "A person with a single mutation will have great difficulty becoming a professional athlete, but they will still be able to go jogging, swimming, and cycling," says Boontanrart, who herself is a carrier of a mutated gene. But if both copies are damaged, the situation becomes problematic: "If you're planning to have children with a partner who also has the mutation, the children might inherit both mutated genes—one from the father, the other from the mother. These children would have a serious illness."
Increasing delta globin production
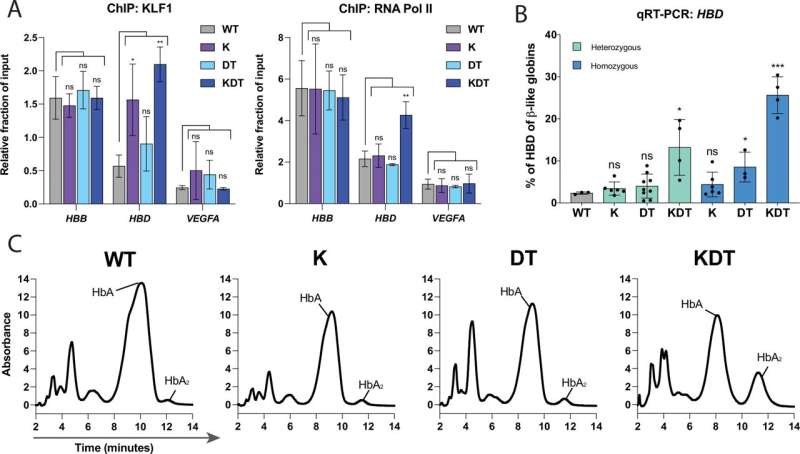
An effective treatment for beta-hemoglobinopathies is yet to be available. In her new study, Boontanrart and her colleagues show that the problem could be solved by increasing production of delta globin, which would replace the faulty beta globin. "Humans naturally produce only tiny amounts of delta globins. This is linked to a special DNA control sequence that hinders the transcription of the relevant gene," Boontanrart says. So the researchers hit upon the idea of altering this control sequence in order to increase delta globin production.
Here, Boontanrart used the CRISPR-Cas9 gene scissors to alter the DNA of progenitor blood cells by inserting three additional sections ahead of the HBD gene, which contains the blueprint for delta globins. These insertions are designed to stimulate the cell machinery to produce more delta globin—and that's exactly what happened.
The results are promising. "We managed to significantly increase in the proportion of delta globin, to the point where it could offer a therapeutic benefit," Boontanrart says.
However, inserting multiple DNA elements is still not without its challenges. "It's more demanding than the techniques used by other research groups and pharmaceutical companies," Boontanrart says. Researchers in the U.S. are also using the CRISPR-Cas9 system to tackle beta haemoglobinopathies by manipulating blood stem cells to produce fetal hemoglobin.
This is the predominant type of hemoglobin found in fetuses, but babies stop producing it at the latest when they are a few months old. For their proposed treatment, the U.S. researchers plan to use fetal hemoglobin to replace beta globin. This approach is currently being vetted for approval by the Federal Drug Administration (FDA).
Although this approach does have relatively broad coverage, it does have its drawbacks, Boontanrart says. It's contraindicated for women who are or are trying to get pregnant, because fetal hemoglobin bonds more strongly to oxygen than adult hemoglobin does. The treatment could therefore result in the mother depriving her unborn child of oxygen.
"In my opinion, increasing delta globin production is the better treatment option. Delta hemoglobin has very similar properties to beta globin and can be used to treat almost all patients," Boontanrart says.
Spin-off in-the-making
To transfer the results of her research into practice, Boontanrart launched the Ariya Bio project in 2021 during her ETH Pioneer Fellowship. Ariya Bio is headquartered in ETH's ieLab in Schlieren on the outskirts of Zurich. The following year, ETH Zurich also applied for a patent to protect the invention.
Boontanrart is now preparing preclinical studies scheduled to start in September. The researchers want to first test their treatment on animals to find out whether it is safe and effective for living organisms. Previous tests were carried out on cell cultures.
Boontanrart says it's too early to tell precisely when her treatment for beta thalassemia will be ready for the market. She hopes to complete all clinical trials and launch a product by 2030. "I'm optimistic the approval process will go faster for us than for the gene editing techniques currently under review, because they're also helping to pave the way for our approach."
More information: Mandy Y Boontanrart et al, Engineering of the endogenous HBD promoter increases HbA2, eLife (2023). DOI: 10.7554/eLife.85258