This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Advancing clinical trial accessibility: A novel software breakthrough in enrollment efficiency

Clinical trials are vital for advancing treatment options to the clinic but often face challenges in patient enrollment. For example, the Cancer Research Institute estimates that only 3% to 6% of eligible cancer patients in the U.S. participate in trials. Patients may be dissuaded from clinical trial participation by accessibility issues, such as distance and transportation costs.
"Finding eligible patients who are willing to participate in and can afford the time and costs required to travel to trial sites is a significant barrier to clinical trial participation," states Jihad Obeid, M.D., professor at MUSC's Biomedical Informatics Center.
The post-pandemic surge in virtual care has inspired a shift towards remote consenting, aligning with patient preferences for convenient, home-based access to treatment, explained Obeid.
Obeid and Clemson University professor Kapil Chalil Madathil, Ph.D., an expert in designing human-machine interfaces, set out to determine which remote consent modality was preferred by patients and study teams. They report their findings in Applied Ergonomics.
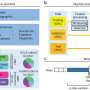
The MUSC-Clemson study compared three approaches: paper-based consent with phone discussion, electronic consent (e-consent) with phone discussion, and a combination of video and audio interactive format with e-consent (teleconsent).
"Each modality was evaluated for task performance and usability to assess its real-world applicability and potential for widespread user acceptance," Chalil Madathil said.
The teleconsent software, designed and developed at MUSC, emerged as the preferred method.
All the remote consenting options eliminated the need for travel, saving time and money for participants, but teleconsent improved real-time communications by enabling direct visual interaction with both patients and researchers. Participants expressed increased trust in the teleconsent platform, citing the benefits of simultaneous review of consent documents and enhanced security through audio/video communication.
"One of the key findings here is that visual cues are critical for effective communication between researchers and research participants," said Obeid. "Teleconsent should improve the capability of a researcher to explain the risks and benefits of a study and more accurately assess the patient's understanding of these things, all important components of the informed consent process."
As with any evolving technology, teleconsent requires ongoing testing and refinement. If it is to make a real impact, it also needs to be affordable and widely available to prevent exacerbating the digital divide. Dedicated community centers could provide computer and internet access for those who need it, Obeid said.
With further study and refinement, remote consent could improve clinical trial enrollment, speeding the advance of needed treatments to the clinic.
"Improving enrollment in general should improve enrollment in clinical trials, particularly problematic in rare disease," said Obeid. "Better remote consenting will also open avenues for multi-institutional clinical trials at remote locations."
More information: Sarvesh Sawant et al, Overcoming recruitment hurdles in clinical trials: An investigation of remote consenting modalities and their impact on workload, workflow, and usability, Applied Ergonomics (2023). DOI: 10.1016/j.apergo.2023.104135