This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
New evidence on cervical cancer screening and treatment for women living with HIV
Following the announcement of the WHO call to eliminate cervical cancer as a public health problem in 2020, there has been encouraging global progress in implementing and expanding Human papillomavirus (HPV) immunization, and cervical cancer screening and treatment programs.
To further support the global strategy for cervical cancer elimination, WHO updated their cervical cancer screening and treatment guidelines in 2021 following an extensive review of the current scientific evidence. In a key shift to the use of high-performance screening tests, WHO recommended the use of primary HPV DNA screening as the main approach for all settings.
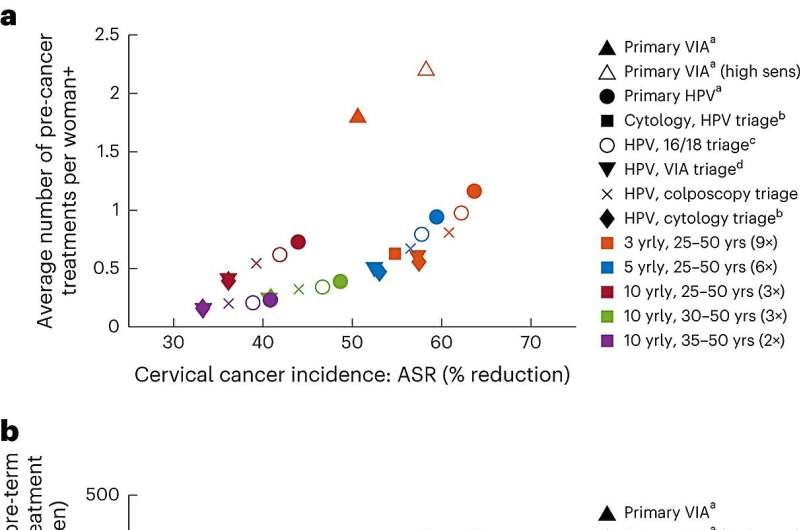
Today, key aspects of the evidence base for the WHO recommendations have been published in Nature Medicine as a pair of papers focusing on both women in the general population, and women living with HIV. These modeled evaluations assessed the benefits and the potential for harm of different approaches with a variety of cervical cancer screening tests (and in combination), screening intervals, and age ranges to start and stop screening.
Separate recommendations were issued for women living with HIV and for women in the general population because of the six-fold increased risk of developing cervical cancer among women living with HIV. The presence of HIV infection weakens the immune system, making individuals more vulnerable to infection with HPV, and also to progression of HPV-related disease.
In recognition of this, the updated WHO guidelines recommended a shorter screening interval of every 3 to 5 years with a primary HPV DNA test for women living with HIV compared to the general population of women, where a screening interval of every 5 to 10 years was recommended. A strategy of primary HPV DNA testing with a second triage test at a 5-yearly interval for women living with HIV was more effective at reducing cervical cancer cases and deaths than screening with visual inspection with acetic acid (VIA) every 3 years.
The inclusion of a second triage test among women living with HIV who screen HPV-positive resulted in minimal loss in efficacy while simultaneously reducing the number of precancer treatments by 11%–52%, depending on the screening technology and interval used. Therefore, WHO recommended implementing an appropriate triaging strategy for women living with HIV (using either HPV16/18 genotyping, colposcopy, cytology, or VIA) to reduce the overall burden in this group.
The development of practical and effective programmatic models of HPV screen-triage-and-treat for women living with HIV will depend on the availability of affordable HPV and triage tests, appropriate linkages with reproductive and HIV services, and effective registry mechanisms for recalling women for follow-up or referring them for further management.
In many settings, bridging strategies will be needed to transition to the infrastructure needed to achieve implementation of these recommendations. During this transition to primary HPV DNA screening, cytology or VIA screening tests should be continued. Successful implementation of the recommended strategies will be critical to both address the substantial burden of disease in women living with HIV, and to improve health outcomes across all women.
"As the public health community comes together across the world to eliminate a cancer for the first time, implementing WHO-recommended cost-effective strategies that allow early identification of women at high risk, reduce burden of cervical cancer, increase equity, and save lives is an essential step. Women living with HIV face many struggles; cervical cancer and the additional burden it causes them should not be one of them," said Dr. Meg Doherty, WHO Director of Global HIV, Hepatitis and STI Programs.
More information: Kate T. Simms et al, Benefits, harms and cost-effectiveness of cervical screening, triage and treatment strategies for women in the general population, Nature Medicine (2023). DOI: 10.1038/s41591-023-02600-4
Michaela T. Hall et al, Benefits and harms of cervical screening, triage and treatment strategies in women living with HIV, Nature Medicine (2023). DOI: 10.1038/s41591-023-02601-3