This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
YBX1 as a key regulator of mitochondrial pyruvate uptake
Cancer metastasis is a crucial area in cancer research that directly affects patient survival and treatment outcomes. Cancer cells often undergo adaptive metabolic changes during metastasis from in situ to distant organs to overcome energy deprivation and achieve rapid proliferation in the changed environment.
Studies have shown that most metastatic tumor cells acquire a higher glycolytic capacity. However, the study of cancer metastasis targets is constrained by the limited understanding of mechanisms regarding cancer metabolic homeostasis.
Recently, Hai-Long Piao lab at Dalian Institute of Chemical Physics, Chinese Academy of Sciences found that the Y-box binding protein 1 (YBX1), a previously known DNA/RNA binding protein, resides in the mitochondrial inter-membrane space (IMS) and interacts with pyruvate transporter protein to inhibit the entry of pyruvate into the mitochondria, which thereby facilitates cancer metastasis.
Their findings, now published in the journal Life Metabolism offer a novel idea for the development of targeted drugs for cancer metastasis.
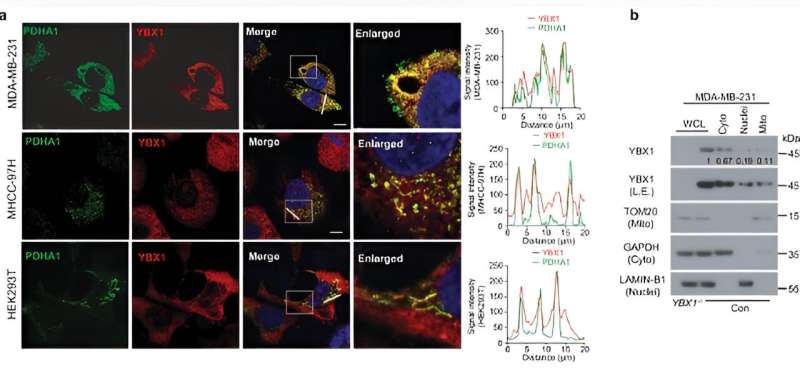
YBX1 is a classical DNA/RNA binding protein that regulates many target proteins highly related to tumor development and metastasis. In the present study, the researchers found that rare YBX1 protein localized to mitochondria in mouse embryonic fibroblast (MEF) cells, whereas more mitochondrial localization of YBX1 protein was in tumor cells as well as in human embryonic kidney HEK-293T cells.
By using protein interaction mass spectrometry, the researchers found that YBX1 in mitochondria interacts with mitochondrial pyruvate carrier (MPC) 1/2, and, by further combining immunoprecipitation and fluorescence resonance energy transfer experiments, they found that the YBX1 protein can bind the MPC1/2 protein and inhibit the entry of pyruvate into the mitochondria through its cold shock structural domain.
Analyzed by stable isotope 13C-labeled glucose and pyruvate tracer technology, the researchers found that downregulation of YBX1 protein in cells promoted the conversion of pyruvate in the mitochondria to produce citrate and alanine as well as oxaloacetate, which increased the cellular oxygen consumption rate and reduced the production of intracellular lactic acid, as well as the dependence of mitochondria on glutamine.
Finally, in tumor cells and mice xenografted with tumor cells, the researchers found that inhibition of the MPC1/2 complex by YBX1 did not alter the proliferative capacity of the cells, but rather promoted the metastatic capacity of the tumor cells.
More information: Huan Chen et al, Mitochondrial YBX1 promotes cancer cell metastasis by inhibiting pyruvate uptake, Life Metabolism (2023). DOI: 10.1093/lifemeta/load038