This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Predicting correct dosage may improve success of drug repurposing
Before a drug can be used to treat a disease, it must go through a lengthy and expensive trial process to prove both safety and effectiveness. By repurposing already-approved drugs, researchers can cut out the time and expense of the former step—sometimes. According to Penn State researchers, repurposed drugs fail as often as novel drugs in clinical trials designed to study efficacy.
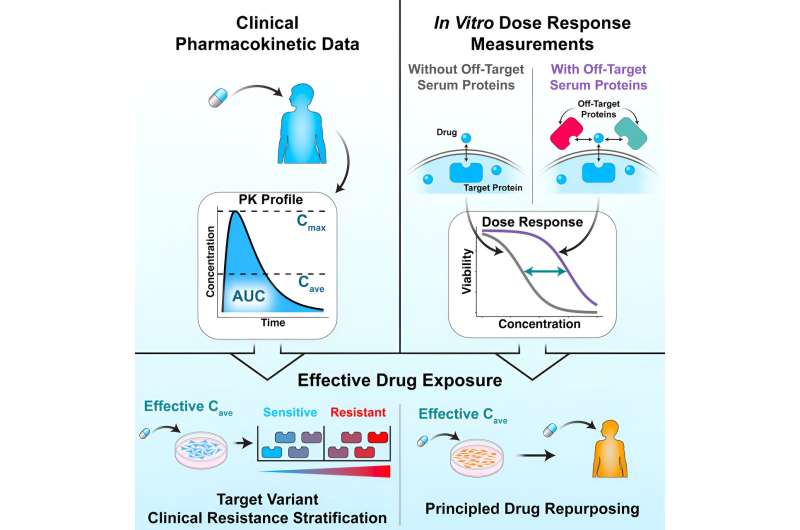
To improve the success rate of drug repurposing and determine effective treatment doses, the Penn State researchers developed a model that predicts effective doses for repurposed drugs. Their results were published in Cell Reports Medicine.
"The nice thing about the drug repurposing process is that you don't have to go back and recheck that it is safe in a patient," said corresponding author Justin R. Pritchard, associate professor of biomedical engineering at Penn State. "But when we actually looked at the numbers, it turned out that just because you got to skip that that first step and cut down on a lot of the costs associated with drug development, the final success rate of these repurposing efforts was about as good as if tried to start from scratch with a new drug."
Paper co-author and Penn State biomedical engineering doctoral researcher Scott M. Leighow said that the low success rate was largely due to an issue with understanding the drug dose in preliminary testing.
"A researcher might think, 'This drug works really well in leukemia. Maybe it'll be a good drug for treating pancreatic cancer or diabetes or COVID,'" he said. "What often ends up happening is that they take that drug and throw enough of it on onto cells in a dish until they see the desired effect and then call it a potential therapy, even though it might be an amount that would not be reasonable to give to a patient."
The other issue, Leighow said, is that cells isolated in a dish may behave differently than cells surrounded by proteins and tissue in a patient's body.
To solve this problem, the researchers developed a computer model using previously existing data on how a drug for leukemia actually performed in real patients. They then fed the model information on their own experimental data of drug-disease interactions for different concentrations on isolated cells in a dish. Specifically, the researchers tested the drug on isolated cancer cells and on cancer cells in a solution of sticky proteins, which exist in blood and keep some of the drug away from its intended target.
By dividing the first number—the percentage of the drug that made it to the target cancer cells in the tests run with sticky proteins—by the second, pulled from the tests run without sticky proteins, they get the serum shift factor. They could then multiply this number by the concentration of drug found in a patient's blood sample to produce a corrected concentration.
"It turns out this is a really good predictor for how well that drug performs in that disease context," Leighow said. "We call this the effective drug exposure in a patient, and it's what lets us translate concentration numbers back and forth between a patient and cells in a dish."
Leighow said they could ask the model for the corrected concentration for a particular drug-disease pairing, which serves as a threshold.
"Experimental performance better than this threshold number we'll call clinically efficacious, and performance worse than this number, we'll say that the drug doesn't work in that context," Leighow said. "We found that we could take that same number that the model gave us and try to evaluate data that the model had never seen before, and ask, 'How well does that same threshold predict if the drug works in this context?' It turned out that that same number performed really well in totally different diseases."
For this research, the team used leukemia data, but when they applied it to lung cancer and gastrointestinal stromal tumors, they saw similar success rates—90% accuracy— and found that they could use similar thresholds to predict drug efficacy.
"Even if that threshold does have a little bit of disease-specific wiggle room, in order for the computer to accurately tell us what that number was, it needed this broader biological context," Leihow said. "We needed to be feeding it the correct numbers, whereas before we weren't."
While this research focused on finding effective doses for repurposing drugs, the researchers said they believe this model could be used for designing new drugs in a similar class in the future.
More information: Chuan Liu et al, Excessive concentrations of kinase inhibitors in translational studies impede effective drug repurposing, Cell Reports Medicine (2023). DOI: 10.1016/j.xcrm.2023.101227