This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Study explains why many cancer drugs fail during clinical trials
Cancer drugs often don't behave as expected in clinical trials. A study published Oct. 11 in the journal Cell Chemical Biology explores why drugs under development are unlikely to work due to insufficient genetic analysis.
Cancer drugs, which are designed to target a single mutated protein and not impact normal tissue, fail for two reasons. "They are too toxic for patients to safely take, or patients can safely take them, but they don't actually shrink a patient's tumor," said the study's senior author Jason Sheltzer, Ph.D., assistant professor of surgery and genetics, and member of Yale Cancer Center. Uncovering the mechanisms behind a mischaracterized drug could shed light on its poor performance in clinical trials.
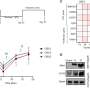
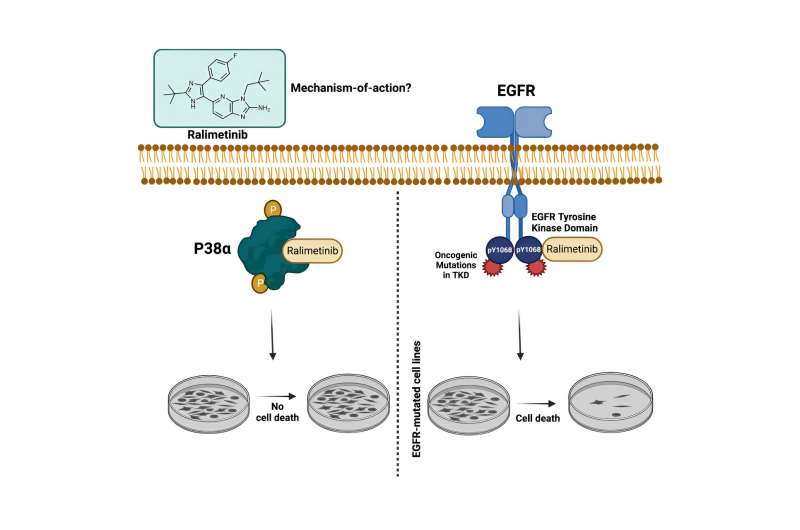
Most cancer drugs fail to receive U.S. Food and Drug Administration approval. To understand why, Debanjan Bhattacharjee, Ph.D., and Jaweria Bakar from the Sheltzer Lab studied a p38a inhibitor drug called ralimetinib.
"When we tested this drug in our lab, we found that it was targeting a different pathway," said Sheltzer. None of the patients responded since the cellular sensitivity to ralimetinib exhibited the strongest correlation with EGFR inhibitors. "If you look at the cancer types where this drug was tested versus the cancer types that are known to be driven by EGFR, there is just no overlap," said Sheltzer.
"Oftentimes, researchers will develop a drug, and they see that the drug kills cancer cells. And that will be enough to motivate a trial. But there's no genetic understanding of how it kills cancer cells or why it kills cancer cells. And without that genetic understanding, you don't actually know which cancer patients to give it to, because you don't know which biomarkers or genetic alterations to look for in a patient's tumor in order to give them that drug," said Sheltzer.
Scientists need a multi-modal approach to target the underlying mechanisms of the disease. "If we had this genetic understanding of the drug sooner, then it might have been given to a different set of patients who would have been more likely to respond," said Sheltzer. Scientists can gather evidence using a combination of tools including CRISPR, pharmacogenomic profiling, and structural evaluation.
Although further testing is needed, a genetics-first approach has broad applications in the fight against cancer. Genetic analysis in a multidisciplinary environment is fundamental for improving the success rate of new therapies.
More information: Debanjan Bhattacharjee et al, Inhibition of a lower potency target drives the anticancer activity of a clinical p38 inhibitor, Cell Chemical Biology (2023). DOI: 10.1016/j.chembiol.2023.09.013