This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Study finds loss of RBFOX2 protein promotes pancreatic cancer metastasis through effects on cytoskeletal remodeling
Pancreatic cancer presents a challenging prognosis, marked by a five-year overall survival rate of merely 12.5%. Diagnosis frequently occurs at an advanced stage, characterized by metastasis to distant sites. The intricate molecular mechanisms governing pancreatic cancer metastasis remain a subject of ongoing research and exploration.
In a recent article published in Nature Communications, Moffitt Cancer Center researchers, in collaboration with The Tisch Cancer Institute; St. Jude Children's Research Hospital; the Agency for Science, Technology and Research in Singapore; and the University of Otago in New Zealand, demonstrate that deregulation of a protein called RBFOX2, involved in RNA splicing, contributes to the progression and metastasis of pancreatic cancer.
RNA is a nucleic acid that promotes the synthesis of proteins. While the nucleotide sequence of RNA is encoded by the DNA of the genome, RNA molecules undergo several modifications before eventually entering the ribosome to make protein. An important process that modifies RNA sequences is alternative splicing, whereby specific sequences within the RNA molecule may be rearranged, included or excluded.
This process results in different versions of RNA molecules generated from a single DNA sequence that will produce protein variants with potentially different activities. Alternative RNA splicing is critical for many cellular processes; however, the involvement of alternative RNA splicing in cancer development is not entirely understood.
The research team wanted to assess whether alternative RNA splicing is involved in the development and progression of pancreatic cancer. Through molecular-based laboratory experiments and mouse models, they discovered that RNA-binding proteins involved in splicing regulation were involved in pancreatic cancer progression. One of the key proteins they identified is RBFOX2.
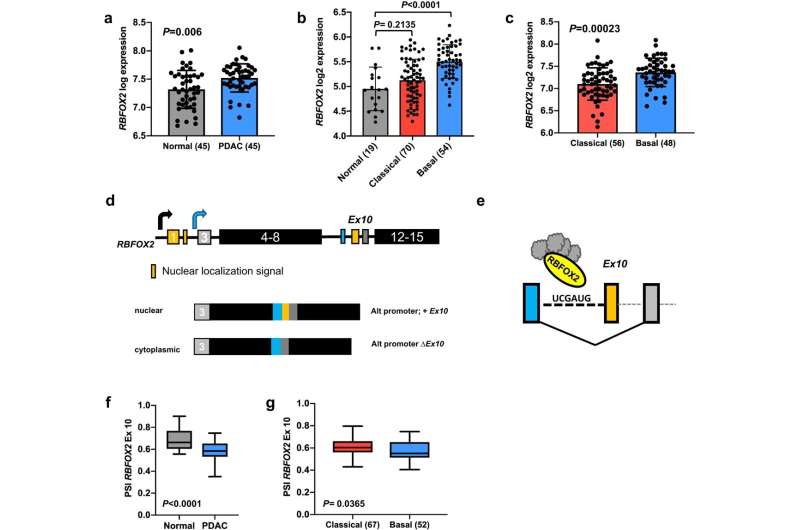
Results showed RBFOX2 protein levels were lower in pancreatic cancer cells that had metastasized to other sites. These observations suggest that RBFOX2 may be a tumor suppressor protein that normally functions to inhibit cancer metastasis. The researchers confirmed this hypothesis by demonstrating that loss of RBFOX2 in pancreatic cell lines and mouse models increased cell migration, invasion, tumor development and metastasis.
The researchers performed additional experiments to determine the downstream pathways impacted by RBFOX2 that lead to pancreatic cancer progression.
They found that RBFOX2 regulates the splicing of RNA molecules that code for proteins involved in cytoskeletal remodeling, with one of the critical molecules being RNA that codes for the protein ABI1. In cells without RBFOX2, the location of ABI1 is redistributed to the cell periphery, where it impacts the cell cytoskeleton and promotes cell migration.
These combined observations demonstrate the importance of alternative RNA splicing to pancreatic cancer progression and suggest the need for additional studies to understand the mechanisms regulating RBFOX2 splicing activity fully.
"While analyses to date have identified few splicing regulators with prognostic implications in pancreatic cancer, the incidence of exon splicing events conserved across pancreatic tumors and other cancers suggests the processes regulated by alternatively spliced transcripts are integral to cancer progression," said Karen Mann, Ph.D., assistant member of the Department of Molecular Oncology at Moffitt.
More information: Michelle Maurin et al, RBFOX2 deregulation promotes pancreatic cancer progression and metastasis through alternative splicing, Nature Communications (2023). DOI: 10.1038/s41467-023-44126-w