This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Study reveals new connection between impaired autophagy and heart failure
A new study sheds light on how autophagy, the body's process for removing damaged cell parts, when impaired, can play a role in causing heart failure.
The research team led by Dr. E. Dale Abel, chair of the Department of Medicine at UCLA, and Dr. Quanjiang Zhang, adjunct assistant professor of medicine at UCLA, identified a signaling pathway that links autophagy to the control of cellular levels of a key coenzyme known as NAD+, which is found in all living cells and is central to how our metabolism works. Researchers say these findings may have implications for heart failure treatment.
"Heart failure remains a leading cause of mortality, and there is a strong need to develop new therapies that can improve cardiac function and increase survival. Identifying a new pathway linking impaired autophagy, a characteristic of heart failure, and increased NAD+ breakdown could open new avenues for therapeutic intervention," Abel said.
Autophagy is a natural, self-serving mechanism in which the body removes damaged or dysfunctional cell parts and recycles their components for cellular repair or energy production. Abel describes it as our body's cellular recycling system that allows cells to break down bad parts of themselves and salvage some of those parts to repurpose into new, usable parts.
Given the important role of autophagy in cell breakdown and repair, impaired autophagy is known to cause a range of disorders, including cancer, neurodegeneration, and heart failure.
Until now, however, scientists weren't sure what mechanisms underlie mitochondrial and cardiac dysfunction in the setting of impaired autophagy. One proposed mechanism has been the build-up of dysfunctional mitochondria that takes place when autophagy is impaired, as this build-up can activate inflammatory and other responses that can lead to cell death or dysfunction.
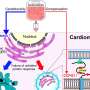
Another proposed mechanism is the degradation of specific proteins involved in metabolism that reduce the functioning of pathways that regulate heart muscle contraction. The present study identified a new mechanism, namely NAD+ depletion, that leads to heart muscle cell dysfunction in the context of autophagy.
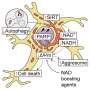
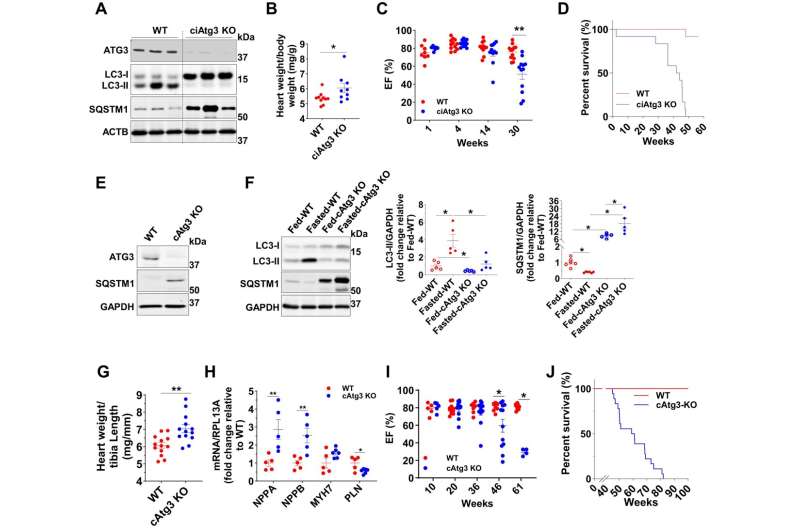
Using a mouse model with autophagy dysfunction in heart cells, the research team found that autophagy regulates the enzyme NNMT, which increases levels in this heart failure model. Inhibiting the activity of this enzyme with a small molecule led to an improvement in heart failure even though autophagy failure persisted.
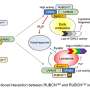
The study elucidated a chain event that explains how and why impaired autophagy can lead to cardiac dysfunction. The first step of this chain is accumulating a protein known as SQSTM1. Increased SQSTM1 activates a signaling protein called NF-κB (also known as RELA). NF-κB enters the nucleus and causes increased gene activity that encodes the nicotinamide N-methyltransferase enzyme (NNMT). NNMT ultimately leads to a breakdown of NAD+ precursors, ultimately lowering NAD+ levels. Low NAD+ levels then cause mitochondrial and cardiac dysfunction.
Unraveling this pathway has linked NAD+ metabolism and autophagy. These findings indicate a new potential therapy for reversing mitochondrial dysfunction and improving heart failure by preventing NAD+ loss and boosting its level in cardiac muscle.
This research is published in The EMBO Journal.
More information: Quanjiang Zhang et al, Control of NAD+ homeostasis by autophagic flux modulates mitochondrial and cardiac function, The EMBO Journal (2024). DOI: 10.1038/s44318-023-00009-w