This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
'Shredding' unique genetic features of cancer cells with CRISPR-Cas3
Cornell researchers have taken an important step toward harnessing CRISPR gene editing in "targeted, safe and potent" cancer treatment, according to Ailong Ke, professor of chemistry and chemical biology in the College of Arts and Sciences.
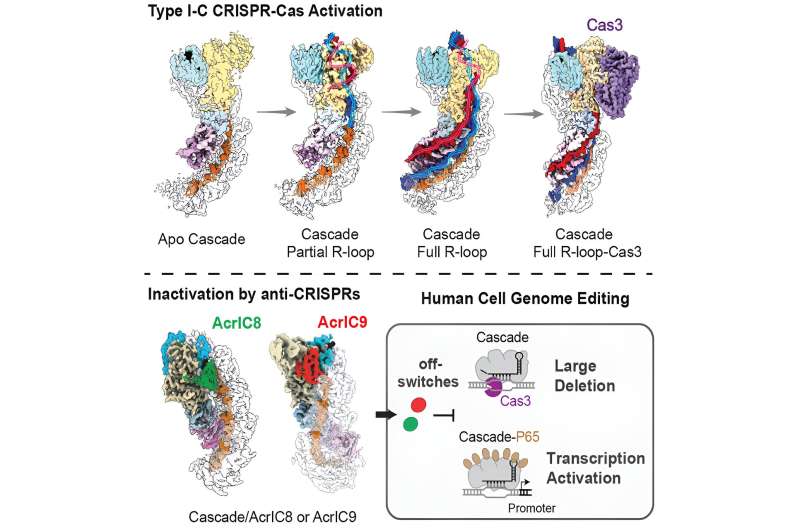
The enzyme CRISPR-Cas3, one of many different CRISPR systems found naturally in the immune systems of bacteria, could ultimately be used to find unique genetic features in cancer cells and shred them, killing the cancer cells while leaving normal cells intact, Ke said. In a collaboration with researchers at the University of Michigan, Ke has uncovered the fundamental mechanisms that drive the CRISPR-Cas3 system and the control of its editing activity inside human cells.
The article, "Exploiting Activation and Inactivation Mechanisms in Type I-C CRISPR-Cas3 for Genome-editing Applications" was published in Molecular Cell on Jan. 18.
The study was a collaboration with the lab of Yan Zhang, assistant professor of biological chemistry at the University of Michigan. First authors are Chunyi Hu, previously a postdoctoral associate in Ke's lab, and Mason T. Myers of the University of Michigan.
"Rather than make a double strand break (in the DNA) and stop there," as the thoroughly studied CRISPR-Cas9 system does, Ke said, "CRISPR Cas3 will shred DNA into pieces. When introduced into human cells, it will cause long-range deletions in the human genome."
Found in nature in bacteria's immune systems, CRISPR can be repurposed to target any DNA sequence, Ke said—that is, once researchers know the sequence that flanks the target DNA, known as a protospacer-adjacent motif (PAM). It's like a street address for DNA.
"In theory [CRISPR] should be able to identify any sequence you want to target in the genome," Ke said. "But in reality only a subset of genomic sequences can be targeted. This is because each Cas enzyme looks for a specific signature sequence near the target, called the PAM sequence. If the therapeutic target lacks the particular PAM that the Cas enzyme is looking for, we are out of luck. To overcome this limitation, we need to collect as many distinct Cas tools as possible."
Ke and other researchers have characterized a few CRISPR-Cas3 tools already, and the current study confirms yet another. They hope to ultimately build an anti-cancer arsenal containing about half a dozen Cas3 tools, each with a unique PAM preference. This way, they can target a broader spectrum of mutations in cancers that have high mutational burdens, he said, such as skin and lung cancers.
The current study focused on a CRISPR system known as I-C, found naturally in the bacteria Neisseria lactamica. I-C targets a PAM different from previously studied CRISPR systems.
The researchers successfully identified the PAM sought by CRISPR-Cas3 system I-C and recorded its mechanisms using cryogenic electron microscopy (cryo-EM). They also found two proteins called anti-CRISPRS that are reliable off-switches for the I-C mechanism.
"We want to harness this power but we want to make sure it's safe to use in patients. We show that I-C Cas3 meets both criteria," Ke said.
The study gives the researchers confidence that they know this molecular system inside and out: how it works, how it picks the target, how it activates the nucleus to degrade the target, and how the activity can be controlled by the anti-CRISPR proteins.
Next, the researchers will apply the Cas3 tools to cancer mutations in cell cultures to study their impact.
While a CRISPR Cas9 gene editing therapy has been approved in the U.S. to treat certain eligible patients with sickle cell disease, research on CRISPR-Cas3 has a long way to go until clinical settings, Ke said. A long-term goal of his lab and many other researchers is to increase the number of genetic diseases that can be treated with CRISPR as various targets are identified.
"There's enormous power in those CRISPR systems and people want to apply it to any genetic disease they want. We're really ambitious, trying to target cancer, which is a very aggressive form of genetic disease," Ke said. "What disease people choose to target now is really determined by the technical limitation of the tool: How safe it is, how impactful it is, how efficiently we can deliver the tool."
More information: Chunyi Hu et al, Exploiting activation and inactivation mechanisms in type I-C CRISPR-Cas3 for genome-editing applications, Molecular Cell (2024). DOI: 10.1016/j.molcel.2023.12.034