This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Viral protein fragments may unlock mystery behind serious COVID-19 outcomes

There are many lingering mysteries from the COVID-19 pandemic. For instance, why does SARS-CoV-2, the virus behind the disease, cause severe symptoms in some patients, while many other coronaviruses don't? And what causes strange symptoms to persist even after the infection has been cleared from a person's system?
The world may now have the beginning of answers. In a study appearing in the journal Proceedings of the National Academy of Sciences, a UCLA-led multidisciplinary research team explores one way that COVID-19 turns the immune system—which is crucial for keeping people alive—against the body itself, with potentially deadly results.
Using an artificial intelligence system they developed, the study authors scanned the entire collection of proteins produced by SARS-CoV-2 and then performed an exhaustive series of validation experiments.
The scientists found that certain viral protein fragments, generated after the SARS-CoV-2 virus is broken down into pieces, can mimic a key component of the body's machinery for amplifying immune signals.
Their discoveries suggest that some of the most serious COVID-19 outcomes can result from these fragments overstimulating the immune system, thereby causing rampant inflammation in widely different contexts such as cytokine storms and lethal blood coagulation.
The study was led by corresponding author Gerard Wong, a professor of bioengineering at the UCLA Samueli School of Engineering and in the UCLA College's chemistry and biochemistry department and microbiology, immunology and molecular genetics department.
"What we found deviates from the standard picture of viral infection," said Wong, who is also a member of the California NanoSystems Institute at UCLA. "The textbooks tell us that after the virus is destroyed, the sick host 'wins,' and different pieces of virus can be used to train the immune system for future recognition. COVID-19 reminds us that it's not this simple.
"For comparison, if one were to assume that after food gets digested into its molecular components, then its effects on the body are over, it would be very liberating; I wouldn't have to worry about the half-dozen jelly donuts I just ate. However, this simple picture is not correct."
The research team found SARS-CoV-2 fragments can imitate innate immune peptides, a class of immune molecules that amplify signals to activate the body's natural defenses. Peptides are chains of amino acids like proteins, only shorter.
These immune peptides can spontaneously assemble into new structures with double-stranded RNA, a special form of a molecule essential for building proteins from DNA, typically found in viral infections or released by dying cells.
The resultant hybrid complex of the immune peptides and double-stranded RNA kicks off a chain reaction that triggers an immune response.
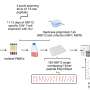
In addition to their AI analysis, the researchers used state-of-the-art methods for elucidating nanoscale biological structures and conducted cell- and animal-based experiments.
Compared to relatively harmless coronaviruses that cause the common cold, the team found that SARS-CoV-2 harbors many more combinations of fragments that can better mimic human immune peptides.
Consistent with that, additional experiments with multiple cell types all consistently show that fragments of the SARS-CoV-2 coronavirus prompt an amplified inflammatory response compared to those from a common cold coronavirus. Likewise, experiments with mice show that fragments from SARS-CoV-2 lead to huge immune response, especially in the lungs.
The findings could influence treatment for COVID-19 and efforts to identify and surveil future coronaviruses capable of causing pandemics.
"We may be able to look at the protein composition of this year's coronavirus strains and figure out whether they're potentially pandemic-capable or just going to cause the common cold," Wong said.
Wong and his colleagues concentrated on three SARS-CoV-2 fragments. Using a technique for analyzing detailed molecular structures called synchrotron X-ray diffraction, they found that, like the innate immune peptide, the SARS-CoV-2 fragments can organize double-stranded RNA into structures that stimulate the immune system.
"We saw that the various forms of debris from the destroyed virus can reassemble into these biologically active 'zombie' complexes," Wong said. "It is interesting that the human peptide being imitated by the viral fragments has been implicated in rheumatoid arthritis, psoriasis and lupus, and that different aspects of COVID-19 are reminiscent of these autoimmune conditions."
The scientists also measured the entire set of genes expressed at the cellular level. By performing a comparison with internationally curated databases, the team found that the gene expression profile from cells exposed to SARS-CoV-2 "zombie" complexes closely resembled that from COVID-19 itself.
"What's astonishing about the gene expression result is there was no active infection used in our experiments," Wong said. "We did not even use the whole virus—rather only about 0.2% or 0.3% of it—but we found this incredible level of agreement that is highly suggestive."
The findings may account for some peculiarities of COVID-19 infection.
For instance, that fragments from SARS-CoV-2 lead to excessive inflammation could help explain why some seemingly healthy people experience severe COVID-19. Normally, the activity of enzymes varies a great deal between healthy individuals—with levels differing by as much as a factor of 10. It is ultimately enzymes that are responsible for cutting virus particles into smaller and smaller pieces.
Evidence that persistence of SARS-CoV-2 fragments may drive illness also reinforces emerging clues about which treatments may show promise.
"Our results suggest we may be able to manage COVID-19 by inhibiting certain enzymes or enhancing others," Wong said. "One could even imagine a strategy also based on mimicry, by using biologically inactive decoys that look enough like these viral fragments to compete for double-stranded RNA, but form complexes that don't activate the immune system."
Remnant viral fragments are known to exist in other viral infections, but their biological activities have not been systematically studied.
The collaborative effort for this study brought together a team with 24 departmental and institutional affiliations during a particularly challenging time of the pandemic. The first author is Yue Zhang, a former UCLA postdoctoral researcher and current assistant professor at Westlake University in Hangzhou, China.
More information: Yue Zhang et al, Viral afterlife: SARS-CoV-2 as a reservoir of immunomimetic peptides that reassemble into proinflammatory supramolecular complexes, Proceedings of the National Academy of Sciences (2024). DOI: 10.1073/pnas.2300644120. doi.org/10.1073/pnas.2300644120