This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Breakthrough in understanding the cause of a rare and life-threatening condition related to sleep apnea
Ben-Gurion University (BGU) of the Negev's Prof. Gad Vatine and Tel Aviv University's Dr. Avraham Ashkenazi are up and coming principal investigators. Vatine is an expert in studying rare disorders using patient-specific stem cells, and Ashkenazi is an expert in tri-nucleotide repeat expansion disorders and protein clearance pathways.
While they don't usually work on the same diseases, the Yad Laneshima patient organization asked them to work together to find treatments for central congenital hypoventilation syndrome (CCHS), which causes young children to stop breathing as soon as they fall asleep. This means that if they fall asleep without ventilatory support, they will suffocate to death.
Prof. Vatine and Dr. Ashkenazi were intrigued by the challenge and began a collaboration that has yielded important new information about the cause of CCHS, which could lead to future treatments. Their findings were published last month in The EMBO Journal.
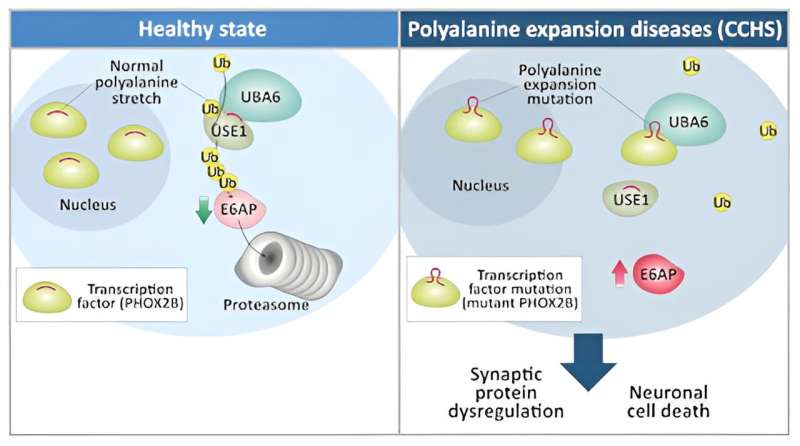
CCHS is caused by a mutation in the PHOX2B gene, a key transcription factor in the development of the autonomic nervous system (ANS), a system that controls non-voluntary body functions such as breathing, digestion and heart rate. PHOX2B and eight other nuclear proteins that cause various neural disorders have a polyalanine tract. In these disorders, a mutation that expands the polyalanine tract causes the disease.
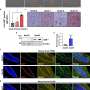
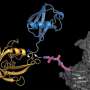
Ph.D. students Fatima Amer-Sarsour from Ashkenazi's lab and Daniel Falik from Vatine's lab identified a polyalanine that is also present in one of the enzymes of the ubiquitin transfer system. Profs. Aaron Ciechanover and Avram Hershko from Israel, along with Prof. Irwin Rose, were awarded the Nobel Prize in Chemistry in 2004 "for the discovery of ubiquitin-mediated protein degradation."
In a healthy condition, this polyalanine stretch is required for enzyme recognition enabling proper ubiquitin transfer to target proteins, such as those involved in neural development, thereby controlling their degradation. In a disease such as CCHS, the Vatine and Ashkenazi research teams discovered that the expansion mutation of the polyalanine tract in PHOX2B (and in other polyalanine disease-causing proteins) causes aberrant interaction with the polyalanine-recognizing enzyme of the ubiquitin transfer system. This interaction disrupts the proper ubiquitin transfer to neural proteins, which inhibits the ubiquitous normal functions, leading to cell death and eventually triggering CCHS.
To make this discovery clinically relevant, the Vatine lab at the Regenerative Medicine and Stem Cell (RMSC) research center at BGU used patient-specific stem cells, termed induced pluripotent stem cells (iPSCs). They used a technique called reprogramming, developed by Japanese Nobel Prize winner Shinya Yamanaka.
This technique enables easily accessible cells (like blood cells or skin cells) to be "taken back in time" to become identical to embryonic stem cells (ESCs). Unlike ESCs, iPSCs are generated without destroying embryos, and can be generated from any individual. The iPSCs from CCHS patients were then differentiated into PHOX2B-expressing cells of the ANS, which revealed the disease mechanism in the most vulnerable of the patient's nerve cells.
Daniel Falik explains, "Using personalized, cutting-edge technologies, we have uncovered insights that can pave the way for significant advances in the disease therapeutics."
Fatima Amer-Sarsour adds, "I am thrilled with the progress we have made in identifying defective pathways in CCHS, as this opens up exciting research avenues to explore the development and function of the ANS."
Prof. Vatine and Dr. Ashkenazi are very excited about the potential of this groundbreaking discovery. "Now that we know what goes wrong in the CCHS patient neurons, we can start developing modalities to fix it with the goal of promoting neuron survival that will allow better quality of life for the patients," they affirm.
More information: Fatima Amer-Sarsour et al, Disease-associated polyalanine expansion mutations impair UBA6-dependent ubiquitination, The EMBO Journal (2024). DOI: 10.1038/s44318-023-00018-9