This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Generative modeling framework helps predict relationship between neural readings and patient symptoms
Over the past decade or so, scientists have amassed an impressive arsenal of weapons to address the multifaceted, complex challenge of mental illness, from new genomic analysis tools and high-resolution neuroimaging technologies, to the creation of huge patient data banks and new artificial intelligence models to analyze them.
Yet despite the emergence of these sophisticated tools, the search for personalized treatment—a medical approach in which practitioners use a patient's unique genetic profile to tailor individual treatment—has so far come up short for nearly every neuropsychiatric disorder.
These efforts have been fundamentally limited by a lack of understanding of how symptoms map onto brain circuits, said John Murray, a former professor of psychiatry and physics at Yale now at Dartmouth College.
A new generative modeling framework developed at Yale, however, can help neuropsychiatrists better predict the relationship between multiple neural readings and patient symptoms, researchers say. This advance can help the field of neuropsychiatry "make informed decisions about how studies and clinical trials are designed and executed to have a higher chance of mapping symptoms to brain circuits," said Alan Anticevic, the Glenn H. Greenberg Associate Professor of Psychiatry and associate professor of psychology and one of the authors of the study.
The findings are published in the journal Communications Biology.
The challenges of finding patient-specific treatments for neuropsychiatric diseases are myriad, researchers say. Psychiatric conditions are highly complex and involve many different regions of the brain. And symptoms can vary greatly among individuals.
While the advent of artificial intelligence models has offered the promise of tailoring new drugs to a variety of individual symptoms for disorders like schizophrenia, studies have shown that while mathematical algorithms were able to predict patient symptoms or outcomes within the specific clinical trials for which they were developed, they failed to work for similar groups of patients participating in a different study.
These divergent results have been blamed on "overfitting"—or the tendency of artificial intelligence models to find patterns of activity in small amounts of data that disappear when applied to other groups of subjects.
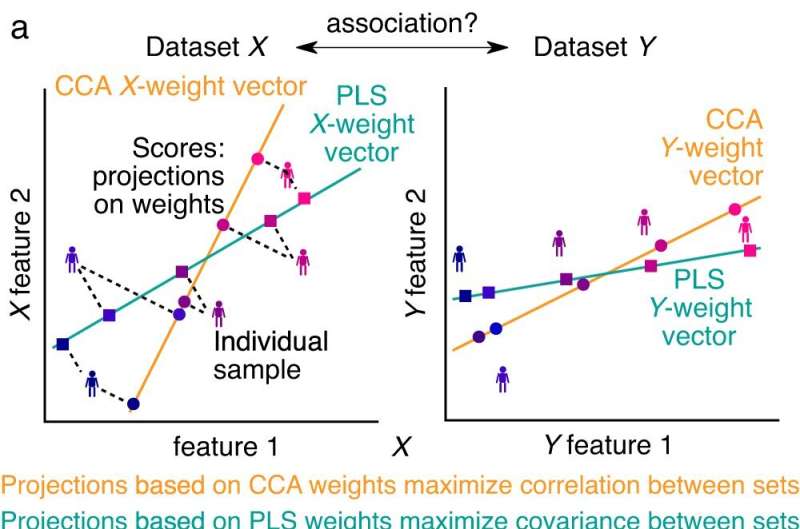
To overcome these limitations, neuroscientist Markus Helmer designed a new statistical generative model while he was a postdoctoral researcher in Murray's Yale lab. The model can estimate the optimal size of data sets needed to accurately estimate how brain signals relate to behavior in different individuals.
In the new study, which was led by researchers at Yale and the University of Nottingham and utilized Helmer's model, scientists looked at neuroimaging data of individuals collected from two large neuroimaging-psychometric data banks—from the Human Connectome Project, a massive project, supported by the National Institutes of Health, that is studying the structural and functional connectivity of the human brain, and the UK Biobank, which contains de-identified genetic information and biological samples from a half-million participants.
The researchers found that to reliably measure an association between multiple neural regions and multiple behavioral traits may require as many as 10,000 individual subjects.
Based on this finding, researchers say the Yale model provides the criteria needed to establish the reliability of brain-symptom relationships, which in turn can be used by drug companies to design and interpret neuroimaging studies that seek to find neurological basis for psychiatric symptoms in diverse groups of patients.
More information: Markus Helmer et al, On the stability of canonical correlation analysis and partial least squares with application to brain-behavior associations, Communications Biology (2024). DOI: 10.1038/s42003-024-05869-4