This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Interactions between flu subtypes found to predict epidemic severity more than virus evolution
Researchers have shed new light on how viral evolution, population immunity, and the co-circulation of other flu viruses shape seasonal flu epidemics.
The research, published today as a Reviewed Preprint in eLife, explores the relationships among evolutionary and epidemiological quantities in influenza and presents fundamental findings that substantially advance our understanding of the drivers of influenza epidemics, according to the eLife editors.
The authors used a rich set of data sources to provide what the editors say is compelling evidence on the roles of genetic distance, influenza subtype dynamics, and epidemiological indicators in predicting flu epidemics. The findings highlight genetic distance and subtype interference as key factors in determining the timing and severity of annual outbreaks.
Influenza viruses continually accumulate genetic changes in two key proteins displayed on their surface—called haemagglutinin (HA) and neuraminidase (NA)—a process known as antigenic drift. Antigenic drift allows the virus to escape immune recognition in people who have been vaccinated or had a previous infection, leaving them exposed to re-infection.
Of the two types of influenza viruses that routinely circulate together in humans (influenza A and B), influenza A, and particularly subtype A(H3N2), has the fastest rates of antigenic drift and causes the most severe illness and death.
Amanda Perofsky, the lead author of the study and a research scientist at the Brotman Baty Institute, University of Washington, US, and Fogarty International Center, National Institutes of Health, says, "In theory, antigenic drift will lead to more susceptible individuals, which in turn should lead to more flu infections and larger or more severe epidemics."
"However, prior evidence for this in epidemiological data has been mixed. Mutations in HA are considered to cause the majority of antigenic drift, so surveillance efforts to monitor and predict influenza evolution focus on HA."
"There has been less focus on NA, even though antibodies against NA also correlate with immunity and influence infection severity. Moreover, outside of flu pandemics, little is known about how different cocirculating influenza subtypes potentially interfere with each other, affecting the size of outbreaks."
In this study, Perofsky and colleagues from the Fred Hutchinson Cancer Center, the Icahn School of Medicine at Mount Sinai, the US Centers for Disease Control and Prevention, and the World Health Organization tracked A(H3N2) evolutionary dynamics—measured by both genetic sequences and serological assays—and linked this to epidemiological flu surveillance data in the US over 22 influenza seasons before the COVID-19 pandemic.
They started out by identifying different indicators of flu evolution each season, using HA and NA genetic sequence data from the Global Initiative on Sharing Avian Influenza Data (GISAID) Epiflu database and serological data on immune cross-reactivity between viruses provided by the World Health Organization Global Influenza Surveillance and Response System (GISRS) Collaborating Centers in Tokyo, London, Melbourne and Atlanta.
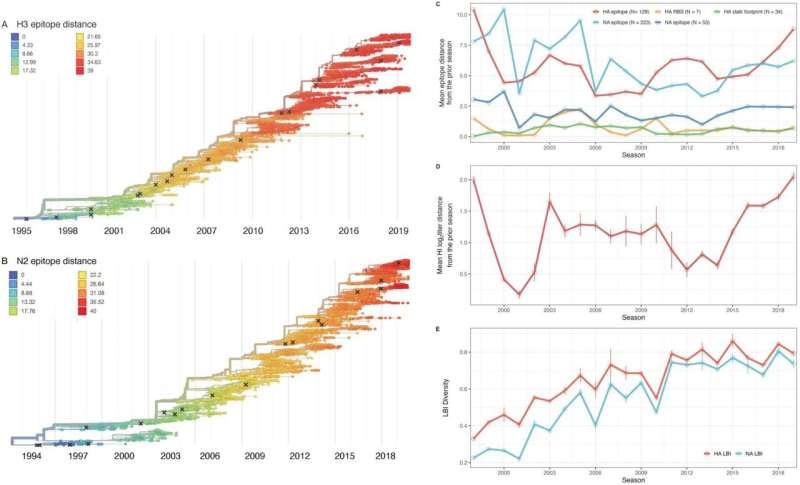
They created phylogenetic trees showing lines of evolution of A(H3N2) viruses over time and measured genetic and antigenic distances between viruses circulating in consecutive seasons to show how far apart the viruses evolved in that timeframe.
Next, the team explored which of these measures of viral 'fitness' are most strongly related to variation across A(H3N2) flu outbreaks in different years.
When comparing the predictive performance of sequence-based and serological evolutionary metrics, they found that genetic distances based on broad sets of antigenic sites ("epitopes") in HA and NA had the strongest, most consistent relationship with the disease burden of annual epidemics, rates of virus transmission, and severity of infection, and influenced the dominant subtype of influenza A—A(H3N2) or A(H1N1)—circulating in each season.
When relating genetic distance in HA versus NA to A(H3N2) epidemiology, the antigenic drift of H3 (the HA antigen of the A(H3N2) virus) was more strongly linked to epidemic size, viral transmissibility, the age distribution of cases, and the number of excess deaths caused by the A(H3N2) virus.
Meanwhile, antigenic drift of N2 (the NA antigen of the A(H3N2) virus) was more strongly linked to the intensity of epidemics (the "sharpness" of the epidemic curve) and greater prevalence of A(H3N2) cases relative to A(H1N1) cases in the population. Increased drift in N2 was also associated with fewer days from epidemic onset to peak incidence (a measure of the speed of the epidemic).
"The direct link between NA antigenic drift and A(H3N2) incidence patterns was a surprising and key result from our study," says Perofsky. "We expected HA to exhibit stronger associations with seasonal incidence, given that it elicits a stronger immune response than NA."
Taken together, the authors' results showed that greater antigenic drift in both HA and NA was linked to larger, more intense epidemics, higher transmission, a greater dominance of the A(H3N2) subtype, and more cases in adults than children.
Finally, the team applied machine learning models to assess the relative importance of viral evolution, prior population immunity, the co-circulation of other flu viruses, and vaccine coverage and effectiveness in predicting regional A(H3N2) epidemic dynamics. Using one of the models (called a random forest), they could accurately predict regional epidemic size, peak incidence, and subtype dominance patterns of seasonal outbreaks that occurred between 1997 and 2019.
Surprisingly, they found that the incidence of A(H1N1) was more predictive of A(H3N2) epidemics than viral evolution, suggesting that interactions between different viral subtypes—that is, subtype interference—play a key role in influenza type A infection dynamics.
"Our study shows that influenza epidemic dynamics are shaped in part by the virus' antigenic drift in both surface proteins, and that genetic sequence indicators of antigenic drift were more informative than serological (or antibody) data for predicting epidemics in the timeframe analyzed," concludes senior author Cécile Viboud, a staff scientist at Fogarty International Center, National Institutes of Health.
"Our work is the first to link NA antigenic drift to epidemic burden, timing, and the age distribution of infections. The connection between NA drift and seasonal incidence further highlights the importance of monitoring evolution of both HA and NA to inform the selection of strains for annual flu vaccines and epidemic forecasting efforts."
More information: Amanda C Perofsky et al, Antigenic drift and subtype interference shape A(H3N2) epidemic dynamics in the United States, eLife (2024). DOI: 10.7554/eLife.91849.1