This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
What archaeogenetics tells us about liver steatosis in ancient and modern humans
Researchers from Würzburg University Hospital (UKW), Homburg University Hospital (UKS), and the Max Planck Institute for Evolutionary Anthropology in Leipzig (MPI-EVA), all in Germany, have analyzed the DNA from a worldwide database of more than 10,000 ancient and modern humans to search for the origin of a mutation in the fatty liver gene PNPLA3 and explain its strikingly high global presence today. The study is published in Gut.
The liver is the central organ in the human body responsible for carbohydrate and fat metabolism. Excess fat deposition in the liver occurs under conditions of overnutrition and ranges from benign steatosis which affects up to 30% of modern populations to an inflammatory, progressive condition termed steatohepatitis. The latter can lead to severe scarring (fibrosis or even cirrhosis) and cancer of the liver.
The deposition of fat in the liver is not only modulated by environmental factors such as nutrition, but also by genetic predisposition. A common variant in the gene PNPLA3 has been identified as being prominently associated with increased risk of developing steatotic liver disease and segregates in present-day human populations, exceeding a 70% frequency in Mesoamerica.
One hypothesis for the wide spread of this variant despite its unfavorable effects on human health is that unfavorable genes involved in metabolism may have evolved for survival in the late Paleolithic era. Particularly the ability to store fat was likely an advantage throughout most of human history but it is now detrimental under modern-day living conditions.
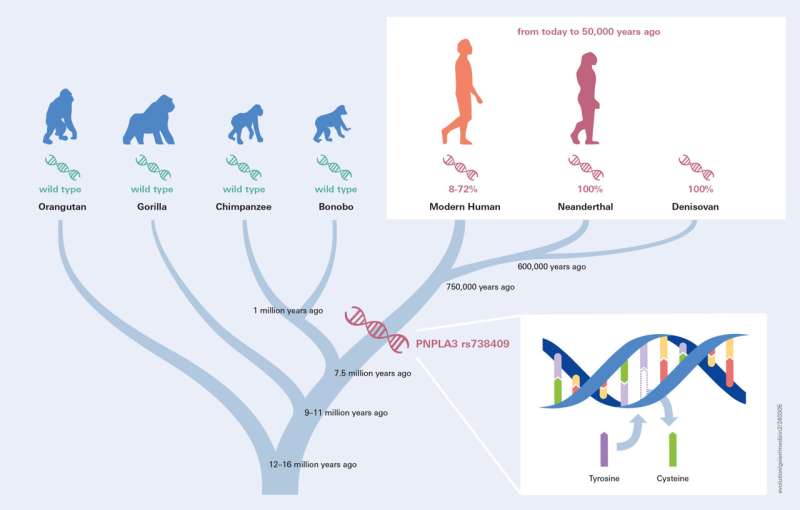
To explore the ancient origins of the PNPLA3 variant rs738409, the researchers first explored the deeper evolutionary context in other species from the human lineage tree. Analyzing the reference genome sequence of primates it became clear that all great apes (Chimpanzee, Bonobo, Gorilla, Orangutan) are actually carrying the ancestral, less risky, gene variant.
Surprisingly, however, all available Neanderthal (total of 21) and Denisovan genomes (two, including the worldwide unique Neanderthal/Denisovan hybrid) dating back to 40,000–65,000 BP exclusively carried the risk allele suggesting fixation of the variant allele in their common ancestor.
These findings led to the conclusion that the principal fatty liver gene variant PNPLA3 must have developed before the split of the human lineage some 700,000 years ago.
The wide range of variant allele frequency in present-day humans ranging from 8% in Kenya up to 72% in Peru poses the question of its trajectory. The researchers first visually inspected the allele frequencies from present day backward through the last 15,000 years, where sufficient ancient genomes in most regions of the world are available to estimate allele frequencies.
Overall, within this relatively recent window of time, distributions of ancestral and variant alleles in modern humans roughly match the distribution we observe today, including a high frequency of the fatty liver risk allele in the Americas even in the earliest samples from around 10,000 years ago.

To quantify changes through time, the team from Würzburg, Homburg and Leipzig finally performed statistical analyses by grouping modern human individuals according to geography and age. Although the frequency of the risk allele decreases over time from 15,000 BP to present, no significant signal for genetic selection could be obtained during this relatively short time window.
What can these archaeogenetic findings tell us about liver steatosis in ancient and modern humans? "One can speculate that our observation underscores a putative advantage of hepatic fat storage in cold climate and particularly for Neanderthals under ice age conditions," explains Prof. Andreas Geier, Head of Hepatology at Würzburg University Hospital (UKW).
Supportive of this hypothesis is recent data from the Yakut population in the coldest northeast region of Russia, where the PNPLA3 variant allele is predominant in almost 90% of the population, as Prof. Marcin Krawczyk, another genetic expert involved in the study from Homburg University Hospital (UKS), Germany, points out. "As a consequence from the present study, functions of PNPLA3 on heat production outside the liver certainly deserve future investigation" concludes Prof. Geier.
Does the absence of a signal of natural selection in the archaeogenetic record ultimately argue against the hypothesis of natural selection during the Paleolithic era? Dr. Stephan Schiffels, Group Leader at the Max Planck Institute for Evolutionary Anthropology (MPI-EVA), advises caution. "While our genome-wide analysis did not detect significant signals of natural selection throughout the last 10,000 years, there is still the possibility of selection being active in time periods older than what we can statistically analyze today."
The most obvious question arising from this study is whether the PNPLA3 variant rs738409 represents genetic heritage (gene admixture) from Neanderthals, particularly since another gene variant in the SLC16A11 locus predisposing to diabetes mellitus and hepatic fat storage has been introduced to modern humans from Neanderthals.
Dr. Schiffels provides a clear answer: "The presence of variant PNPLA3 in African populations argues against introgression from Neandertals into modern humans as the main source of this variant in contemporary populations although it may have contributed to it."
Indeed, subsequent analyses at the Max Planck Institute for Evolutionary Anthropology in Leipzig indicate, that 1 out of 1,000 contemporary PNPLA3 variant alleles may have been derived from the Neanderthal genome.
All involved researchers agree that this joint research project between medical and archaeogenetic experts allows important new insights into the evolutionary basis of human metabolic diseases and reveals unexpected physiological aspects of long known genetic variants.
More information: Andreas Geier et al, PNPLA3fatty liver allele was fixed in Neanderthals and segregates neutrally in humans, Gut (2024). DOI: 10.1136/gutjnl-2023-331594