This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Exploring the effectiveness of a novel pain management device for endometriosis pain
Endometriosis is a chronic condition affecting women, often resulting in painful symptoms such as menstrual cramps and pelvic pain. Pain caused by endometriosis significantly lowers the quality of life and reproductive health of affected women, with around one-third of women still experiencing pain and discomfort despite treatment. While hormonal therapies and surgeries are common treatments, they often do not result in complete alleviation of symptoms.
Effectively managing pain is, therefore, crucial for managing the profound impact of the condition on daily life and work productivity, with challenges persisting even after treatment.
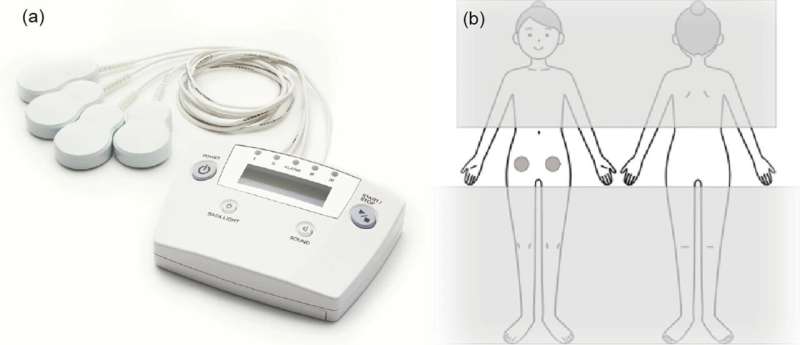
Now, in a recent study published in Reproductive Health, researchers investigate the effectiveness and safety of a new portable pain relief device known as the Angel Touch device (AT-04). The device, developed by Peace of Mind Co., Ltd. in Japan, emits a mix of magnetic fields that target pain in specific areas.
Previous research on animals has shown that the device works by adjusting nerve growth factors, reducing inflammation, and activating the body's natural pain relief mechanisms. Building upon this existing body of knowledge, Associate Professor Hiroshi Ishikawa and Professor Kaori Koga from Chiba University's Graduate School of Medicine, along with their team, are now undertaking a study to investigate the potential of AT-04 in managing endometriosis-related pain by utilizing its unique combination of alternating magnetic fields.
"We focused our study on AT-04 since it is an exceptionally minimally invasive device suitable for premenopausal women, as it does not disrupt ovulation. Its method of pain management differs significantly from current treatments, indicating promising potential for effectively addressing endometriosis-related pain," says Dr. Ishikawa, underscoring the efficacy of AT-04 in managing endometriosis-related pain.
Approved by the Clinical Study Review Board, this study involves premenopausal women above the age of 18 with moderate to severe endometriosis-related pain. Fifty participants will be randomly divided to either receive genuine electromagnetic wave treatment from AT-04 or use a placebo device for a "double-blind" period lasting 16 weeks.
Following this, both groups will wear the AT-04 for an additional four weeks. The researchers then aim to measure changes in pain levels using a pain scale and also look at other factors such as pelvic pain, participants' quality of life, and safety.
In addition to assessing pain levels using the Numeric Rating Scale (NRS), the study also evaluates the participants' Health-Related Quality of Life (HRQoL) using the Endometriosis Health Profile-30 and the EuroQol 5-Dimension scoring systems. These tools can provide insights into various aspects of HRQoL, including pain, emotional well-being, and social support.
Despite the potential benefits of AT-04, the study still has several limitations which require further consideration. For instance, determining the device's sole efficacy in treating endometriosis-related pain can be challenging since most participants would have already undergone treatment for the condition.
Furthermore, hormonal fluctuations associated with the menstrual cycle can impact pain and HRQoL assessments during the research period. Additionally, the effectiveness of pain relief by AT-04 can vary among participants with moderate pain levels.
Despite these limitations, however, Dr. Ishikawa is optimistic about the future potential of this study. Elaborating further, he says, "Women experiencing persistent endometriosis-related pain often endure mental strain such as depression. This stress, alongside the condition itself, can exacerbate future fertility issues."
"By effectively managing long-term pain with minimal side effects using AT-04, the device is expected to enhance the quality of life for women with endometriosis-related pain and also to potentially safeguard against future declines in fertility."
In summary, the potential of AT-04's benefits offers hope for those enduring persistent endometriosis-related pain unresponsive to existing treatments. Promisingly, this study underscores physicians' commitment to enhancing reproductive health and quality of life for women battling this condition.
More information: Hiroshi Ishikawa et al, Efficacy and safety of a novel pain management device, AT-04, for endometriosis-related pain: study protocol for a phase III randomized controlled trial, Reproductive Health (2024). DOI: 10.1186/s12978-024-01739-8