This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Immune evasion tactics of liver cancer cells revealed
A faulty cancer gene helps tumors evade immunity by stopping tumor cells releasing message-containing cargoes called exosomes. These findings, published in eLife, provide what the editors say is fundamental insight into the underlying biology of hepatocellular carcinoma (HCC), and compelling data supporting the role of beta-catenin in immune evasion.
They suggest this work will be of special interest to researchers investigating the basic biology of liver function, as well as those investigating novel pathways for therapeutic targeting of liver cancer.
HCC is the most frequent form of primary liver cancer, accounting for around 80% of cases. It is one of a subset of tumors caused by faulty beta-catenin genes. These beta-catenin-activated tumors—which also include bowel, womb and melanoma skin cancer—all tend to have tumor microenvironments containing few immune cells. This means that immunotherapy treatments which rely on unleashing an influx of immune cells into the tumor will not work. This is a type of treatment resistance known as "immune escape."
"As immunotherapies are now the first-line treatment for HCC and other cancers, it's important to better understand the mechanisms underlying immune escape," says co-author Violaine Moreau, research scientist at the University of Bordeaux, INSERM, France. "Exosomes—small vesicles that transport messages between cells—are known to control infiltration of immune cells into the tumor microenvironment, but their regulation by beta-catenin in tumor cells has not been explored."
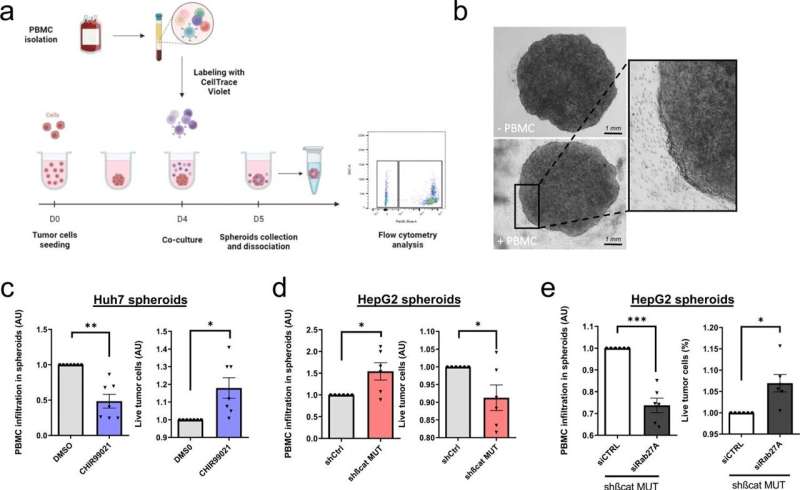
To investigate this, the team studied the effects of silencing the mutated beta-catenin gene on liver cancer cells. They first identified two exosome-associated genes that looked to be influenced by the faulty beta-catenin gene—called syndecan-4 and Rab27A. Next, they used a nanoparticle tracking method and a fluorescent label to monitor the release of exosomes within liver cancer cells.
When mutant-beta-catenin was silenced, they saw an increase in the number of exosomes released. This led them to speculate that in tumor cells where mutant beta-catenin is active, it might reduce the release of exosomes carrying important signals within the tumor microenvironment.
Next, they tested whether silencing the activity of mutant-beta-catenin would affect immune cell infiltration, by using 3D tumor spheroids from two different liver cancer cells lines. This showed that when faulty beta-catenin was silenced, not only did it enhance the infiltration of immune cells into the tumor spheroids, it was also associated with increased tumor cell death. This suggests that silencing mutant beta-catenin could be a viable therapeutic strategy for triggering the influx of immune cells into these hard-to-treat tumors.
To explore whether this mechanism might hold true in patients, the team verified whether human liver cancer samples had dysregulated levels of the exosome-associated genes, syndecan-4 and Rab27A. As hoped—in two independent sets of human liver cancer samples—the levels of these genes were markedly reduced when beta-catenin was faulty. Moreover, other markers of beta-catenin activity were also linked to low levels of Rab27A protein in the liver tumor samples.
"Our study is the first description of the cancer gene beta-catenin as a key regulator of exosome release and provides new insights into control of tumor-derived exosome production and its involvement in immune escape," concludes co-author Clotilde Billottet, a Professor of Cell Biology at the University of Bordeaux, INSERM.
"Given that beta-catenin signaling is also activated in melanoma, womb and bowel cancers, these new findings may have broad significance for cancer immunotherapy. Our results suggest that exosomes could be an important marker of treatment response, and that restoring tumor exosome release in beta-catenin-mutated tumors could increase tumor-to-immune cell communication, and make immunologically 'cold' tumors, 'hot.'"
More information: Camille Dantzer et al, Emerging role of oncogenic ß-catenin in exosome biogenesis as a driver of immune escape in hepatocellular carcinoma, eLife (2024). DOI: 10.7554/eLife.95191.1