March 29, 2024 feature
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
An infamous 'inflammasome'—a rogue protein complex—appears to underlie a rare and disabling autoimmune disorder
Autoimmune diseases are among the most puzzling because turncoat constituents of the body wage a constant state of war. Sometimes the underlying cause of an autoimmune condition is so obscure—hidden within chemical miscues of the body—that a long investigatory search must be mounted to sleuth out a cause.
And so it has been with in-depth research to understand an extremely rare autoimmune disorder. The condition is known as CAPS—cryopyrin-associated periodic syndrome. It afflicts patients with an array of disparate symptoms, ranging from skin rashes to permanent hearing loss.
To understand the disorder, scientists have had to explore the influences of cellular biomechanics and the roles of chemical miscues linked to an infamous inflammasome, a protein complex that triggers extreme inflammatory activity. CAPS, scientists now say, occurs because of an inflammasome that's gone rogue.
Inflammasomes are multi-protein complexes found in the cytosol of cells that rapidly assemble and activate proinflammatory signaling in response to a diverse number of stimuli.
Normally, inflammasomes guard us against infection and cancer by triggering the domino effect of a powerful immune response. But inflammasome activity can also go awry and cause uncontrolled inflammation. Indeed, conditions known as autoinflammatory disorders, like CAPS, can occur when the body creates an immune response without an easily discernable cue, leading to a host of debilitating lifelong symptoms.
Like CAPS, there are other rare autoinflammatory conditions that cause a range of symptoms, from skin rashes to devastating inflammatory responses leading to fever, blindness, deafness, and cognitive decline.
Writing in the journal Science Immunology a collaborative team of researchers at multiple institutions in France sought to find out why—and how—a key inflammasome can careen out of control and produce rare, disabling symptoms.
CAPS, the French team says, is related to the overactivation of a notorious inflammasome—NLRP3—which can be influenced by miscues in mechanical signaling. Mechanical signaling occurs when cells in close contact with each other send errant signals that move through the cytoskeletons of affected cells.
The new research describes how mechanical signaling involving immune cells' membrane proteins can lead to autoimmunity affecting patients with CAPS. The rare disorder, which usually begins in infancy, is marked by waxing and waning lifelong symptoms. It's characterized by rashes, joint pain, red eyes and severe headaches with vomiting. Hearing loss usually occurs during the teen years and is often permanent.
Yet, it has taken until now to figure out the underlying molecular and cellular signaling miscues that lead to full-blown CAPS. Key immune system cells—those of the myeloid family—are also involved in the autoinflammatory condition, scientists have found.
"Immune cells sense the microenvironment to fine-tune their inflammatory responses. Patients with cryopyrin-associated periodic syndrome—CAPS—caused by mutations in the NLRP3 gene, develop autoinflammation," writes Dr. Li Ran, lead author of the new inflammasome research.
Ran is an immunologist at the Institut de Génétique et de Biologie Moléculaire et Cellulaire of Université de Strasbourg in Illkirch, France. The NLRP3 complex, whose gene Ran referred to, is the notorious cell-death inflammasome, which can trigger proinflammatory activity via multiple types of stimuli.
NLRP3 apparently becomes dangerous when its proinflammatory activity can't be switched off. When this happens, it's like having an "on switch" permanently flipped to the "on" position. In addition to CAPS, a mutated NLRP3 inflammasome complex also underlies certain neurogenerative disorders, such as Alzheimer's disease, arthritis, some forms of cancer, and gout.
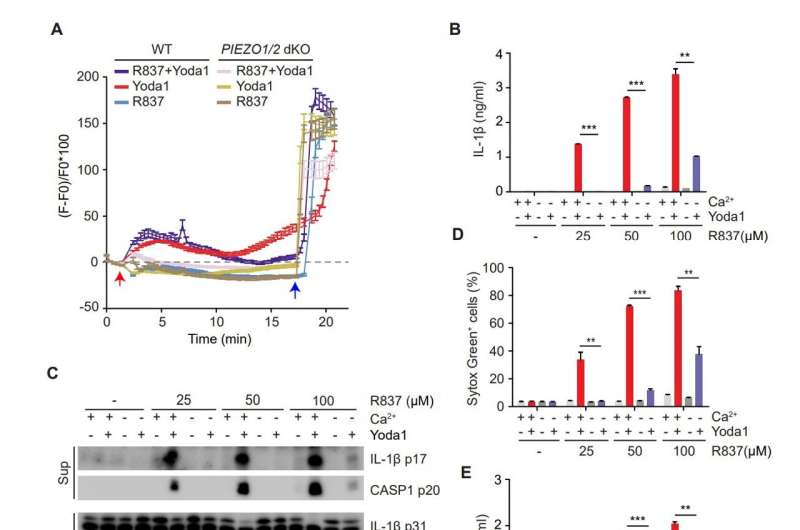
Ran and colleagues explored some of the many ways a process called mechanotransduction, mediated by the protein PIEZO1, might contribute to the disease-generating activity of NLRP3, allowing CAPS to flourish. Inflammasomes, including the NLRP3 complex, trigger proinflammatory activity through multiple types of chemical stimuli, the scientists found.
In the lab, the team zeroed in on a triggering protein called PIEZO1 and studied its impact on the NLRP3 inflammasome in human macrophages and, additionally, in a mouse model. In the lab studies, the French collaborators discovered that PIEZO1 not only affected NLRP3 but a calcium-activated channel called KCNN4 in myeloid cells, ultimately culminating in an uptick in potassium efflux. This, in turn, propelled NLRP3 inflammasome activation.
But most notable in the lab research was that the team found a way to circumvent the problematic out-of-control proinflammatory activity. Inhibiting the KCNN4 channel in both the human cell lines and the mouse model circumvented inflammasome-triggered autoimmunity.
"Targeting the PIEZO/KCNN4 pathway may provide new therapeutic strategies for the treatment of CAPS patients and potentially other NLRP3-related inflammatory diseases," Ran concludes, noting that these findings might also offer new insights into the control of gouty arthritis, another inflammasome-mediated disease.
More information: Li Ran et al, KCNN4 links PIEZO-dependent mechanotransduction to NLRP3 inflammasome activation, Science Immunology (2023). DOI: 10.1126/sciimmunol.adf4699
© 2024 Science X Network