This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Instructing iPS cell-derived mesenchymal stem cells to inhibit abnormal bone formation in FOP
A team of researchers led by Associate Professor Makoto Ikeya (Department of Clinical Application) has observed early signs of success from providing iPSC-derived mesenchymal stem/stromal cells (iMSCs) instructions to produce an inhibitory molecule to suppress abnormal receptor-mediated signal activation responsible for fibrodysplasia ossificans progressiva (FOP) pathogenesis. Their report is published in Stem Cell Research & Therapy.
FOP is a rare autosomal-dominant genetic disorder characterized by congenital bone malformation and progressive extraskeletal ossification in connective tissues. A gain-of-function missense mutation in the ACVR1 gene (c.617G>A; p.R206H), leading to aberrant BMP signal activation, was identified in 2006 as the underlying cause of this devastating disease.
Ikeya's team previously demonstrated the ACVR2B-Fc fusion protein as a new means to suppress aberrant ACVR1 activation, which was subsequently shown by an independent U.S.-based research group to prevent heterotopic ossification in FOP model mice. However, therapies based on ACVR2B-Fc are not readily available due to high costs.
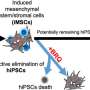
Thus, Ikeya and his team recently devised an alternative strategy in which healthy iMSCs were instructed to produce and secrete the ACVR2B-Fc fusion protein. After verifying that these modified healthy iMSCs produced and secreted the fusion protein, the research team confirmed that it inhibited intracellular BMP and TGF-β signaling as expected.
In addition, they also found conditioned medium (containing the ACVR2B-Fc fusion protein) collected from the modified healthy iMSCs to have similar beneficial effects on FOP patient-specific iMSCs. Through these experiments, the researchers clearly demonstrated in vitro efficacy of ACVR2B-Fc fusion proteins.
In a previous study, the researchers showed that they could reproduce FOP pathology by injecting cardiotoxin (CTX) into the skeletal muscles of mice expressing the ACVR1R206H mutant protein. Therefore, to examine the potential of ACVR2B-Fc-producing iMSCs as a viable therapeutic option for treating FOP, they introduced control (healthy) or ACVR2B-Fc-producing iMSCs, either locally or systemically via intraperitoneal injections into ACVR1R206H mutant mice after intramuscular CTX treatment.
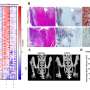
Whereas X-ray or micro-computed tomography imaging revealed heterotopic bone formation at the site of CTX injection in mice not injected with any cells or control iMSCs, locally or systemically administered ACVR2B-Fc-producing iMSCs inhibited aberrant bone formation at the injury site. Furthermore, ACVR1R206H mutant mice with local administration of ACVR2B-Fc-producing iMSCs showed improved movement compared to other treatment groups, as demonstrated by enhanced treadmill performance.
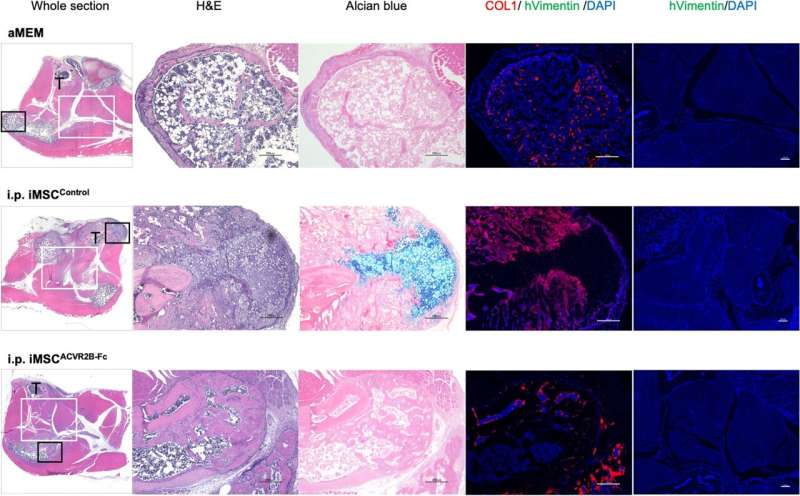
Because surgical procedures such as those intended to resect abnormally formed bones can lead to recurrent heterotopic ossification, the researchers also established a surgical model of FOP to examine whether ACVR2B-Fc-producing iMSCs would have beneficial effects.
They performed surgeries on ACVR1R206H mutant mice to remove abnormal bone formation three weeks following the initial CTX injection, then introduced control or ACVR2B-Fc-producing iMSCs into the animals by intraperitoneal injection to avoid triggering further damage and heterotopic ossification at the site of injury.
Although no significant effects were observed, mice with systemic administration of ACVR2B-Fc-producing iMSCs showed a trend for improved treadmill performance.
Through these experiments, the research team demonstrated the potential therapeutic effects of ACVR2B-Fc-producing iMSCs for primary but not recurring heterotopic ossification. Further optimization of the treatment protocol or additional genetic modifications of the iMSCs designed to enhance their survival following transplantation may augment their successes in the future.
More information: Pan Gao et al, iMSC-mediated delivery of ACVR2B-Fc fusion protein reduces heterotopic ossification in a mouse model of fibrodysplasia ossificans progressiva, Stem Cell Research & Therapy (2024). DOI: 10.1186/s13287-024-03691-7