This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Rare inflammatory disease responds best to double inhibition, shows study

Hemophagocytic lymphohistiocytosis (HLH) is a rare and often aggressive syndrome of hyperactive inflammation with up to a 40% mortality rate. Scientists at St. Jude Children's Research Hospital have shown that a drug inhibiting two major inflammatory signaling proteins works better than drugs inhibiting either protein alone in models of HLH. The drug ruxolitinib, which inhibits both inflammation-related signaling proteins Janus Kinase 1 (JAK1) and JAK2, was superior to other drugs tested, which inhibited only JAK1 or JAK2.
The findings are published in Blood.
"We showed ruxolitinib is a safe and potentially more effective therapy for HLH than the current standard of care," said corresponding author Kim Nichols, MD, St. Jude Division of Cancer Predisposition director, Department of Oncology.
HLH presents as severe and dangerous hyperinflammation. Patients' immune systems overproduce powerful immune-signaling molecules called cytokines. These cytokines further drive the immune response, leading to a positive feedback loop of continued cell proliferation and hyperactivation. One common cytokine, interferon-gamma, is well-known to use JAK1 signaling to cause hyperinflammation in HLH.
In addition to JAK1, ruxolitinib inhibits JAK2. Inhibiting both could potentially cause a dangerous lowering of blood counts, characterized by reduced levels of red and white blood cells and platelets. Lack of these cells puts patients in danger of anemia, infection and excessive bleeding, respectively. The St. Jude group tested three different drugs to see whether inhibiting JAK1 alone, JAK2 alone or both simultaneously increased survival in mouse models.
"We found that to treat our HLH models effectively, you need to inhibit both JAK1 and 2," Nichols said. "That's important because targeting JAK2 has some potential side effects. However, our results further support our continuing efforts with the dual inhibitor ruxolitinib."
Pausing a problematic pair of JAKs with ruxolitinib works best
The researchers investigated two models, one representing hereditary HLH, which usually affects infants and young children, and one representing non-genetic disease, affecting patients at older ages. Findings showed that of the three drugs, ruxolitinib increased survival the most in both model systems. In addition, problematic cytokine production decreased the most in the mice treated with ruxolitinib. While ruxolitinib worked well in both models, the JAK1-exclusive inhibitor only improved survival in non-genetic disease. The JAK2 inhibitor did not meaningfully increase survival in either model.
"Selective JAK1 inhibition works to a certain extent in both HLH models, but not as well as ruxolitinib," Nichols said. Notably, although ruxolitinib also inhibits JAK2, it did not lower blood counts, and it appeared critical to ensure the most robust treatment effect.
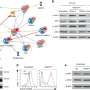
To understand the mechanisms underlying these findings, Nichols and her team performed RNA sequencing using immune cells obtained from the hereditary HLH model following treatment with ruxolinib or the selective JAK1 or JAK2 inhibitors. They observed that ruxolitinib exerted the greatest impact on gene expression, leading to a profound reduction in the expression of genes that normally regulate cell proliferation, metabolism and proinflammatory signaling pathways.
Given these new data, the evidence for ruxolitinib is such that it supports an ongoing clinical trial.
Moving ruxolitinib into clinical trials
Before ruxolitinib, the standard of care for HLH included treatment with a chemotherapy drug known as etoposide. Etoposide, especially in pediatric patients, can have many poor long-term impacts. Ruxolitinib has already been approved for another condition and has demonstrated safety in mice and humans. As a more targeted drug, it can circumvent the whole-body effects seen with chemotherapy.
"That's been my ultimate goal for almost a decade," Nichols said. "To find a better treatment where you didn't have to use a potentially damaging chemotherapeutic agent for kids with HLH. These kids don't have cancer."
St. Jude is now testing ruxolitinib in patients. "We are currently enrolling patients in an ongoing clinical trial to treat HLH using ruxolitinib and the steroid dexamethasone," Nichols said. "In this trial, etoposide is only used for the sickest children who do not respond sufficiently to ruxolinib and dexamethasone. HLHRUXO is a trial designed to see how effective this dual inhibition approach is in treating patients."
The study's first author is Camille Keenan, St. Jude. The study's other authors are Silvia Alemán-Arteaga, University of Salamanca; Lauren Meyer, University of Washington; Sabrin Albeituni, Ninad Oak, Alexa Stroh, Heather Tillman, Yingzhe Wang, Burgess Freeman, Rolanda Woods, Katherine Verbist, Yinmei Zhou and Cheng Cheng, St. Jude.
More information: Camille Keenan et al, Differential Effects of JAK1 vs. JAK2 Inhibition in Mouse Models of Hemophagocytic Lymphohistiocytosis, Blood Journal (2024). DOI: 10.1182/blood.2023021046