This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Rethinking drug efficacy: Research aims to improve drug development
ASPIRE to Innovate Postdoctoral Fellow Catherine Leasure is the co-author of a comment article published this month in Nature Reviews Bioengineering addressing the pressing obstacle faced by modern drug development: worryingly poor success rates of pharmaceuticals progressing to clinical phases.
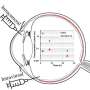
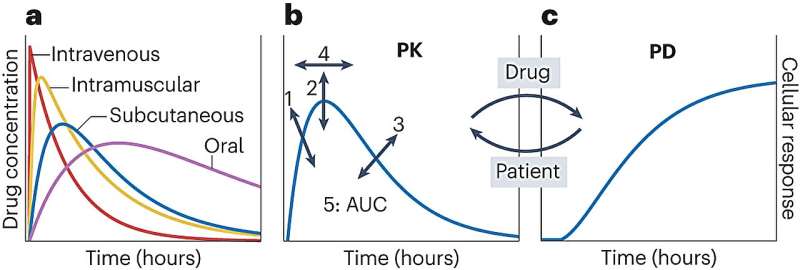
She and corresponding author Gregor Neuert, associate professor of molecular physiology and biophysics, pharmacology, and biomedical engineering, indicate that this problem is partly because dose-response experiments conducted during the pre-clinical phase do not accurately mimic the fluctuating conditions of drug exposure in humans, resulting in incorrect forecasts of a drug's effectiveness and safety.
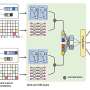
"We propose to approach pharmacokinetic profiles as independent variables that drive drug response, rather than as dependent outcomes of a drug's absorption, distribution, metabolism, and excretion properties," Leasure said. "This paradigm shift allows for the engineering of PK profiles to induce specific pharmacodynamic responses, offering a more accurate replication of in vivo environments. This thinking could help create drugs that are safer and more effective because they are tested in ways that more closely mimic how they will behave in the human body, potentially reducing the failure rate of drugs in clinical trials."
The ideas presented in this article have the potential to transform the drug manufacturing sector by boosting the likelihood of new medications succeeding, cutting expenses linked to drug development and providing health care, diminishing the need for animal experiments, and hastening the delivery of therapies for numerous illnesses. Ultimately, it is poised to enhance patient health results and foster the progress of precision medicine, guaranteeing that treatments are more customized and efficacious.
"We hope that other researchers will build on this work by applying the approach to different therapeutic areas and exploring the implications for drug formulation and delivery," Leasure said.
Neuert and Leasure, who was named the second ASPIRE to Innovate Postdoctoral Fellow in 2023, are developing a startup with technology that will allow drug developers to test the impact of pharmacokinetic profiles on drug responses. "Beyond altering the current drug development procedures, the technology has potential to facilitate the creation of entirely novel drug categories in the future," Neuert said.
"It also enables the investigation of drug mechanisms of action in ways not achievable with any other method, thereby enhancing our understanding of the basic science behind drug mechanisms. The technology will benefit both drug development and fundamental scientific research."
More information: Catherine S. Leasure et al, Modelling patient drug exposure profiles in vitro to narrow the valley of death, Nature Reviews Bioengineering (2024). DOI: 10.1038/s44222-024-00160-x