This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Unlocking the secrets of lung fibrosis with a new mouse model
A RIKEN-developed mouse model of an enigmatic lung disease promises to unlock new biological insights and catalyze the development of treatments for millions affected globally. The research is published in Nature Communications.
Idiopathic pulmonary fibrosis (IPF) is a progressive lung disease in which scarring of the lungs makes breathing increasingly difficult. The cause is unknown with no cure, and it often leads to eventual death.
About a decade ago, Kazuyo Moro of the RIKEN Center for Integrative Medical Sciences and her colleagues investigated the role that a special population of immune cells, known as group 2 innate lymphoid cells (ILC2s), play in the body's response to lung infections. As part of that effort, they created mice lacking two key immune-related genes.
Without these genes, the mice exhibited defective signaling of a critical immune-modulating molecule called interferon-gamma, leading to enhanced activity of ILC2s and the associated inflammation that can spur allergic reactions.
But intriguingly, these same mice tended to develop fibrotic lesions in their lungs as they aged.
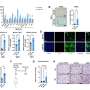
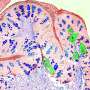
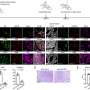
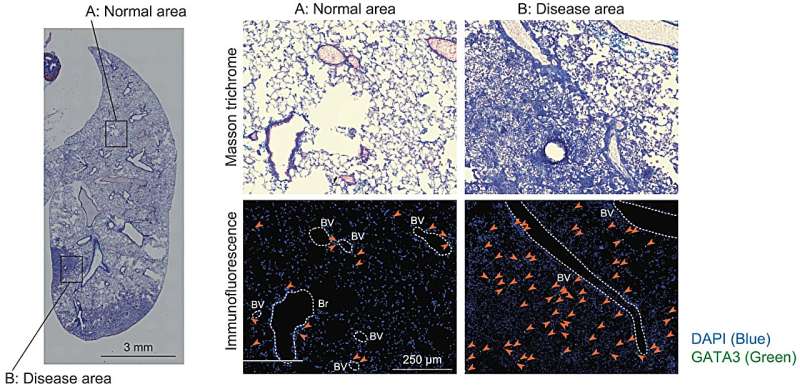
Now, Moro's team has shown that these mice mimic the progression of IPF more closely than other mouse models of the lung disease. In particular, unlike traditional mice models—where lung damage originates within the airways—these mice exhibit scarring on the lung's lining (pleural side), mirroring the pathology observed in IPF patients.
This unique aspect of the model offers unprecedented insights into IPF's external triggers and progression. "This is important for understanding why fibrosis begins in IPF patients," says Moro. "Many basic researchers and pharma companies can now use this mouse model for drug development."
Moro's team showed that the lack of interferon-gamma signaling in these mice results in over-activation of ILC2s. These cells, in turn, express a receptor on their surface that promotes interactions with fibroblast cells on the outside of the lungs, leading to excess collagen production that can spur lung stiffness and tissue thickening.
Supporting evidence for the mouse data came from an interrogation of ILC2s isolated from the blood of IPF patients. As with mice, the patient-derived ILC2s exhibited elevated expression of the receptor needed to engage fibroblasts, along with decreased levels of a protein implicated in interferon-gamma signaling.
The researchers also showed that ILC2-activated fibroblasts initiate the production of IL-33, thereby reactivating ILC2s and setting up a positive feedback loop.
While these mice aren't the perfect stand-in for IPF, they are more reflective of IPF than any commonly used model today, Moro says.
The main drawback is that mice take about 15 weeks to develop signs of fibrosis, longer than other models. Nonetheless, the benefits of biological accuracy far outweigh the convenience of speed, Moro contends.
More information: Natsuko Otaki et al, Activation of ILC2s through constitutive IFNγ signaling reduction leads to spontaneous pulmonary fibrosis, Nature Communications (2023). DOI: 10.1038/s41467-023-43336-6