This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers describe tools to better understand CaMKII, a protein involved in brain and heart disease
The health impacts of a complex protein that plays a major role in the development of Alzheimer's disease and heart conditions can be lessened by three kinds of drug inhibitors, according to scientists at the University of Colorado Anschutz Medical Campus.
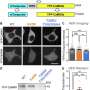
In an overview of the protein and the inhibitors published in Cell Reports, the CU researchers discussed the best ways to use the interventions.
The protein CaMKII is ubiquitous in cells throughout the body but is perhaps best known for its prominent role in the brain and the heart. It is critical in learning and memory, but if misregulated, it can cause problems.
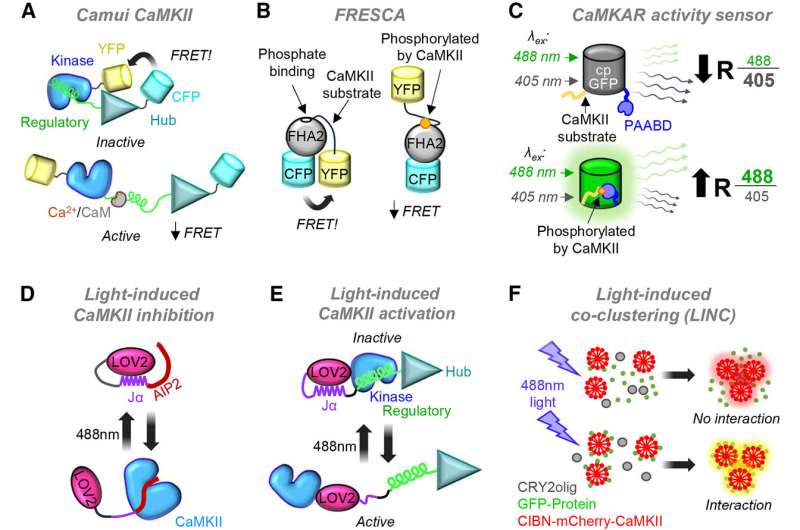
"The most powerful engine to drive new discoveries on CaMKII functions may lie in the availability of three distinct classes of pharmacological inhibitors," said the manuscript's senior author, Ulli Bayer, Ph.D., professor of pharmacology at the University of Colorado School of Medicine. "These inhibitors now allow a detailed first assessment of CaMKII functions in any given system in a way that is readily accessible to a broad range of scientists without specialized interest in CaMKII research."
Carolyn Nicole Brown, a graduate student working in Bayer's laboratory, co-authored the manuscript.
The drugs now allow a detailed first assessment of CaMKII functions in any given system that's accessible to a wide range of scientists.
Previous studies by Bayer's lab revealed that inhibiting CaMKII activity protects against some of the effects of amyloid-beta (Abeta) plaques in the brain, a hallmark of Alzheimer's disease (AD).
The researchers found one group of inhibitors, or drugs, that protected from the Abeta effects without detrimental side effects, making it potentially useful in treating a number of brain diseases.
Yet CaMKII is present in nearly every other cell. The review offers insights into the protein for those who don't study it fulltime, providing tools to fill in the gaps in knowledge about how the protein functions.
"We are experts in studying this complex protein and here we provide a guideline for non-specialists to use these new tools," Bayer said. "We are trying to make it easier for everyone."
Brown, the co-author, agreed.
"The most important advances will be filling the gaps that we don't even know about yet," she said.
More information: Carolyn Nicole Brown et al, Studying CaMKII: Tools and standards, Cell Reports (2024). DOI: 10.1016/j.celrep.2024.113982