This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Difficulties of setting efficacy endpoints in early-stage clinical trials of brain tumor drugs
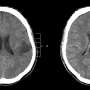
Clinical trials for cancer drugs have three stages, Phase I–III, before marketing, each with different evaluation items. However, there is no consensus on the appropriateness of evaluation criteria for early-stage clinical trials of brain tumor drugs compared with drugs that treat other solid tumors because brain tumors require unique biomarkers and complex evaluations.
Therefore, the appropriateness of criteria for evaluating therapeutic agents is less well understood for brain tumors than that for other solid tumors.
A new study published in Therapeutic Innovation & Regulatory Science analyzed the endpoints in recent Phase I trials of brain tumor drugs. The researchers found that multiple efficacy endpoints, such as overall response rate, progression-free survival, and overall survival, were evaluated in an exploratory manner.
In addition, the Response Evaluation Criteria for Solid Tumors were used more frequently than other criteria in a statistical Phase I examination cohort including brain tumors and other solid tumors.
These findings indicate that evaluating the efficacy of therapeutic agents is more difficult in early-stage examinations of brain tumors than in those of other solid tumors, requiring multidimensional evaluation criteria and the accumulation of detailed prognostic data.
To collect evidence based on the WHO brain tumor guidelines, the efficacy endpoints in early trials must be further analyzed using information in various databases.
More information: Shinya Watanabe et al, Recent Status of Phase I Clinical Trials for Brain Tumors: A Regulatory Science Study of Exploratory Efficacy Endpoints, Therapeutic Innovation & Regulatory Science (2024). DOI: 10.1007/s43441-024-00644-3