This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Simulations reveal mechanism behind protein buildup in Parkinson's disease
Researchers have used computational models to understand what drives the accumulation of alpha-synuclein protein, a key culprit in the development of Parkinson's disease.
The study, published today as a Reviewed Preprint in eLife, is described by the editors as providing important biophysical insights into the molecular mechanism underlying the association of alpha-synuclein chains, which is essential for understanding the development of Parkinson's disease. The data analysis is solid, and the methodology can help investigate other molecular processes involving intrinsically disordered proteins (IDPs).
IDPs play important roles in the human body. These proteins lack a well-defined 3D structure, which allows them to function in a flexible way, adopting different roles as needed. However, this also makes them susceptible to irreversible aggregation, especially if mutated. These aggregates are known to be associated with various diseases, such as neurodegenerative diseases, cancer, diabetes and heart disease.
For example, Alzheimer's disease is characterized by the aggregation of amyloid-beta protein, whereas Parkinson's disease is linked with the build-up of alpha-synuclein.
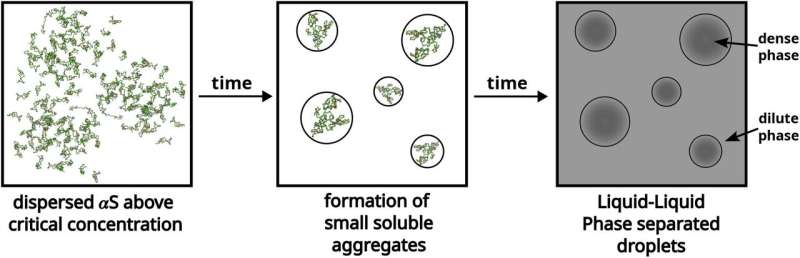
"A growing body of evidence has established a connection between intrinsically disordered proteins and liquid-liquid phase separation, or LLLPs, the phenomenon you see if you mix oil and water," says lead author Abdul Wasim, a Ph.D. student at the Tata Institute of Fundamental Research, Hyderabad, India. "This is of interest because LLPS is itself known to form subcellular compartments that can lead to incurable diseases."
It is known that alpha-synuclein can undergo LLPS, and that the aggregation of alpha-synuclein is influenced by crowding from nearby molecules and surrounding pH. However, characterizing the precise interactions and dynamics of these minuscule aggregate proteins is challenging.
"Previous attempts have simulated individual IDPs, but these simulations can be extremely time-consuming and resource-intensive, making the study of protein aggregation impractical even with cutting-edge software and hardware," explains senior author Jagannath Mondal, Associate Professor at the Tata Institute of Fundamental Research. "We used coarse-grained molecular dynamic simulations, which—although offering lower resolution—allowed us to study the aggregation of multiple IDPs in a mixture."
Using this model, the authors simulated the collective interaction of many alpha-synuclein chains within droplets under different conditions. First, by studying the protein chains mixed only with water, they found that around 60% of the protein chains remained free and did not show a strong and spontaneous tendency to aggregate together.
Next, they added in some "crowder" molecules—large biological molecules that make the environment a highly crowded space for proteins. Previous studies in Alzheimer's disease have shown increased aggregation of proteins in a crowded environment. As expected, the addition of crowders led to enhanced alpha-synuclein aggregation and the number of free proteins decreased.
Similarly, the team found that changing the ionic environment by adding salt also promoted aggregation. However, further exploration revealed that these two environmental factors—crowding and salt—caused aggregation by different mechanisms. Adding salt into the mix increased the surface tension of the droplets, but adding in crowder molecules had no surface tension effects.
This is important to know because the larger the surface tension, the higher the tendency of proteins to aggregate. Moreover, the merging of droplets to alleviate surface tension is often seen in liquid-liquid phase separated (LLPS) droplets, characteristic of diseases involving disordered proteins.
A characteristic of LLPS is that the protein molecules within droplets adopt an extended shape and all orient themselves in a consistent direction. So, the team next set out to see if this was true within their simulations.
They found that proteins in the dense (highly concentrated) phase of the liquid-liquid separation indeed had an extended shape, irrespective of whether crowder molecules or salt were present—all protein molecules had similar orientations—suggesting that alpha-synuclein IDPs display the hallmarks of the LLPS phenomenon.
Next, the team wanted to find out how different alpha-synuclein proteins interact with each other to achieve these effects. By studying the position and features of different amino acids within the protein, they could work out the chances of them coming into contact under different conditions. This revealed that certain amino acids in the protein probably exist to prevent aggregation—and that proteins orient themselves to minimize interactions between these residues.
The editors note that there are limitations to the study to be addressed. Namely, they say that benchmarking of the simulations against other methods could be improved to give the reader greater confidence in the conclusions presented.
"Together, these results suggest that both crowder molecules and salt enhance the aggregation of alpha-synuclein, while also stabilizing the resulting aggregates," says Wasim. "Irrespective of the factors causing the aggregation, the interactions that drive the formation of droplets remain the same."
"Our study focused on normal alpha-synuclein and identified key sites within the protein that are crucial for aggregation," concludes Mondal. "Inherited mutations in alpha-synuclein are thought to increase the likelihood of aggregation significantly. These mutations, involving minor alterations to protein sequence, highlight the importance of understanding the molecular basis of this process."
More information: Abdul Wasim et al, Modulation of α-Synuclein Aggregation Amid Diverse Environmental Perturbation, eLife (2024). DOI: 10.7554/eLife.95180.1