This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Update on the STING signaling pathway in developing nonalcoholic fatty liver disease
Nonalcoholic fatty liver disease (NAFLD) has become the most prevalent chronic liver condition worldwide, affecting about 25% of the global population due to the increasing rates of obesity and metabolic syndrome. NAFLD encompasses a spectrum of liver conditions ranging from simple hepatic steatosis to nonalcoholic steatohepatitis (NASH), which can progress to fibrosis, cirrhosis, and hepatocellular carcinoma (HCC).
Despite its prevalence, there are limited effective treatment options available. Inflammation driven by metabolic disturbances is a key factor in the development and progression of NAFLD. Recent research has highlighted the role of the stimulator of interferon genes (STING) signaling pathway in mediating hepatic inflammation and metabolic disorders, making it a potential target for therapeutic intervention.
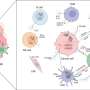
The pathogenesis of NAFLD is closely linked to the innate immune system. Hepatic lipid accumulation leads to oxidative stress, endoplasmic reticulum (ER) stress, and autophagy dysregulation in hepatocytes.
These stressed hepatocytes release damage-associated molecular patterns (DAMPs), which are recognized by pattern recognition receptors (PRRs) on liver-resident immune cells such as Kupffer cells. This recognition activates the immune cells, triggering the release of pro-inflammatory cytokines and chemokines that recruit additional immune cells, including neutrophils, monocytes, natural killer (NK) cells, and NKT cells, to the liver.
This immune cell infiltration exacerbates hepatic inflammation and injury. Moreover, gut microbiota dysbiosis and increased intestinal permeability allow bacterial products such as lipopolysaccharides (LPS) to enter the liver through the portal vein, further stimulating inflammatory responses via Toll-like receptors (TLRs).
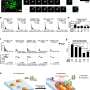
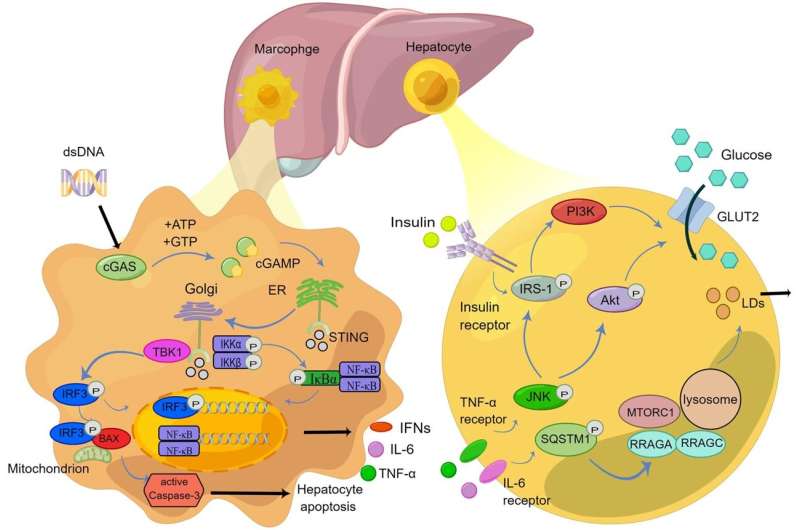
STING is a critical regulator of the innate immune response to cytoplasmic DNA. Located in the ER, STING is activated upon binding to cyclic dinucleotides like cyclic GMP-AMP (cGAMP), produced by the enzyme cyclic GMP-AMP synthase (cGAS) in response to cytoplasmic DNA detection. Activated STING translocates to the Golgi apparatus, where it recruits and activates TANK-binding kinase 1 (TBK1), which in turn phosphorylates interferon regulatory factor 3 (IRF3).
Phosphorylated IRF3 dimerizes and translocates to the nucleus, inducing the expression of type I interferons and other pro-inflammatory cytokines. The STING pathway also activates the NF-κB signaling cascade, further promoting inflammation.
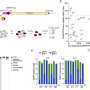
The STING pathway's role in NAFLD progression is multifaceted. High-fat diet-induced NAFLD has been associated with increased expression of STING in liver macrophages and hepatocytes. This elevation in STING activity leads to enhanced production of pro-inflammatory cytokines and chemokines, contributing to hepatic inflammation and fibrosis.
Mitochondrial DNA (mtDNA) released from damaged hepatocytes can also activate the STING pathway, exacerbating liver injury and promoting lipid accumulation. In NASH, STING activation has been shown to influence lipid metabolism by increasing the expression of enzymes involved in lipid synthesis while decreasing those involved in lipid breakdown, leading to hepatic steatosis.
Given its central role in mediating hepatic inflammation and metabolic disturbances, the STING pathway presents several therapeutic targets for NAFLD. Pharmacological inhibitors of STING, such as C176, have demonstrated efficacy in reducing liver inflammation and improving metabolic parameters in preclinical models.
Targeting STING interactions with various immune cells, including macrophages, dendritic cells, NK cells, and T cells, offers new avenues for drug development. Modulating these pathways can potentially ameliorate hepatic inflammation, insulin resistance, and lipid dysregulation, which are critical in NAFLD pathogenesis. Furthermore, targeting the cGAS-STING pathway might not only alleviate liver-specific symptoms but also address systemic metabolic dysfunctions associated with NAFLD.
The STING signaling pathway is a pivotal component in the inflammatory and metabolic disturbances observed in NAFLD. Its regulation of innate immune responses and involvement in metabolic pathways underscore its significance in disease progression.
Developing therapeutic strategies targeting STING and its associated immune mechanisms holds considerable promise for managing and potentially reversing NAFLD. Continued research into STING inhibitors and their effects on liver inflammation and metabolism is essential to address the growing global burden of NAFLD effectively.
The paper is published in the Journal of Clinical and Translational Hepatology.
More information: Wei Liu et al, Update on the STING Signaling Pathway in Developing Nonalcoholic Fatty Liver Disease, Journal of Clinical and Translational Hepatology (2023). DOI: 10.14218/JCTH.2023.00197