This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New insights on cellular clones and inflammation in bones
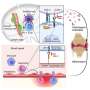
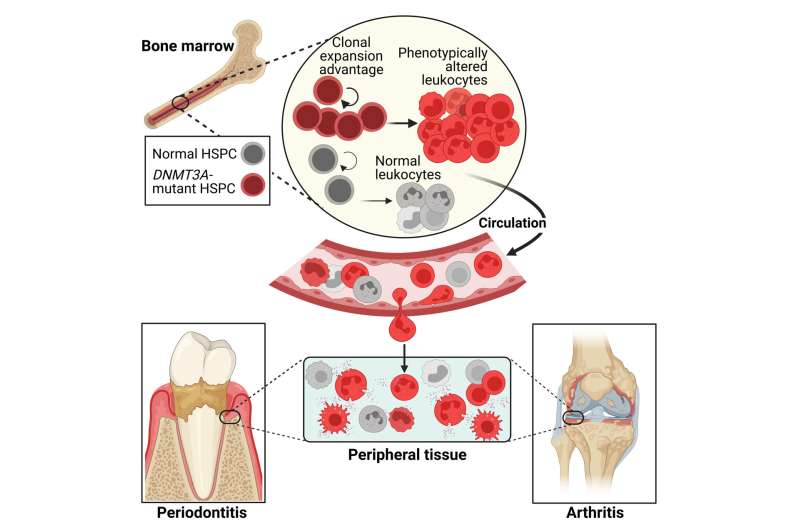
As humans age, hematopoietic stem cells—the immature precursor cells that give rise to all blood and immune cells—accumulate mutations. Some of the mutations allow these stem cells to self-renew and expand more effectively than their non-mutated counterparts.
This relatively poorly understood condition, known as clonal hematopoiesis of indeterminate potential (CHIP), is detectable in more than 10% of people older than 65 and is linked to increased risks of various inflammation-related diseases.
"These mutations change the character of the progeny cells, making them more inflammatory," says George Hajishengallis of the University of Pennsylvania's School of Dental Medicine. "When a large fraction of your immune cells are derived from these mutant stem cells, it spells bad news for chronic inflammatory diseases."
Now, a team led by Hajishengallis, together with collaborators at the Dresden University of Technology and the University of North Carolina at Chapel Hill (UNC), have uncovered mechanistic insights into CHIP. They also found that an FDA-approved drug for preventing organ transplant rejection, rapamycin, has the potential to block these mutant stem cells and treat CHIP-driven inflammatory bone loss diseases, such as periodontitis and arthritis. Their research is published in the journal Cell.
"We found a compelling observational association between DNMT3A, a gene most commonly affected in CHIP, and the prevalence and severity of periodontitis in a cohort of 4,946 people aged 52 to 74," Hajishengallis says.
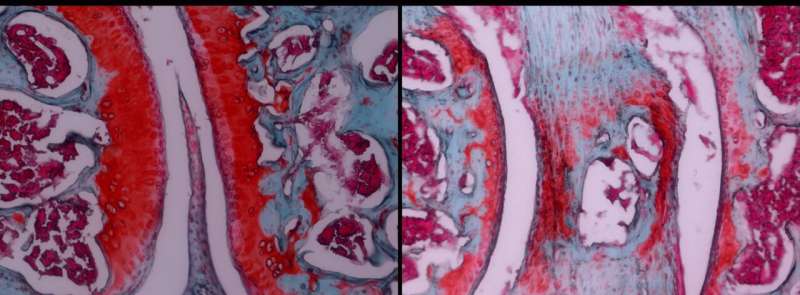
"What's more, we corroborated these findings with our mouse model, demonstrating a strong causal relationship between DNMT3A mutations and increased susceptibility to inflammatory bone loss disorders. And most excitingly, we were able to show the efficacy of rapamycin in protecting mice from CHIP-exacerbated inflammatory bone loss, which paves the way for eventually treating such diseases in humans."
"CHIP and the pathological mechanisms that we described in this work have implications for several aging-related inflammatory disorders, which emerge as comorbidities," says Triantafyllos Chavakis of the Dresden University of Technology, a co-senior author of the study.
The initial motivation for this research stemmed from the observation that CHIP was linked to cardiovascular disease, which prompted Hajishengallis and postdoctoral researcher Hui Wang, co-first author of the study, to hypothesize that CHIP might also be associated with other inflammation-related conditions.
Hajishengallis and Wang reached out to collaborators at UNC, who were then able to investigate this association in a large, community-based study of periodontitis and heart disease, another inflammatory condition driven by CHIP.
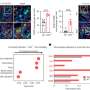
The UNC researchers studied the association between DNMT3A mutations and the prevalence and severity of periodontitis and gingival inflammation. They did this by analyzing genetic data and clinical characteristics of the 4,946 community-dwelling study participants.
Kimon Divaris, co-first author of the study, says this analysis "provided robust epidemiological evidence from a sizeable community-based cohort," supporting their hypothesis that CHIP could be linked to inflammatory bone loss disorders, which prompted development of the animal study.
The Hajishengallis lab's animal model had a mutation analogous to a common human DNMT3A mutation found in CHIP.
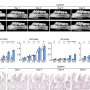
"This revealed that mice with the DNMT3A mutation developed periodontitis naturally and experienced worsened symptoms when periodontitis and arthritis were experimentally induced," Wang says.
These mutations led to an increase in cells that break down bone tissue, higher levels of a protein involved in inflammation, and impaired function of regulatory T-cells, which normally keep the immune response in check.
The presence of the DNMT3A mutation also resulted in overactive signaling of mTOR (mechanistic targeting of rapamycin)—which regulates cell growth, proliferation, and survival—in the hematopoietic stem and progenitor cells with CHIP mutations.
This overactivation contributed to the expansion of these mutant clones' descendants and the heightened inflammatory response. Based on literature related to mTOR signaling, the researchers knew that inhibiting this pathway could be a potential therapeutic strategy.
Considering potential therapeutic strategies Hajishengallis says, "Screening for CHIP among the elderly population may identify individuals with increased risk for inflammatory comorbidities."
These individuals may benefit from therapeutic interventions aiming to block the aberrant expansion of the CHIP-mutant hematopoietic stem cell clones and their adverse impact on chronic inflammatory comorbidities.
More information: Hui Wang et al, Clonal hematopoiesis driven by mutated DNMT3A promotes inflammatory bone loss, Cell (2024). DOI: 10.1016/j.cell.2024.05.003