This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Intestinal organoids reveal the mechanism of gastrointestinal motility
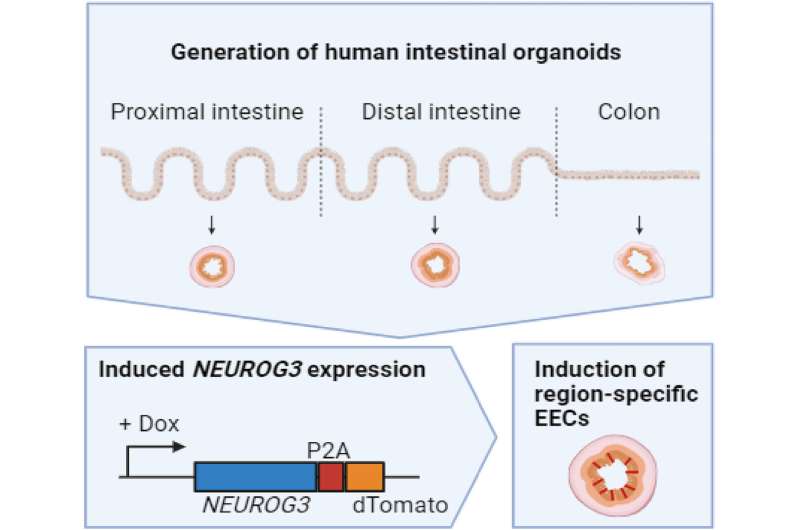
A new study, led by Professors Mashaghi and Clevers from Leiden University and Hubrecht Institute in the Netherlands, has introduced a novel approach using intestinal organoids to uncover the mechanism of gastrointestinal motility. The study presented the first single-cell mechanical characterization of human Enteroendocrine cells (EECs) isolated from healthy intestinal organoids.
The work is published in the journal Mechanobiology in Medicine.
Using single-cell optical tweezers, the researchers measured EEC stiffness profiles at the physiological temperature and investigated changes following tryptophan metabolism inhibition. These findings not only shed light on EEC mechanics but also highlight the potential of adult stem cell-derived organoids for studying these elusive cells.
The main role of the gastrointestinal (GI) tract is to digest food. This process relies on coordinated fluid secretion and bowel movement in response to mechanical stimuli in the gut. The GI tract senses these stimuli through a process called mechanosensation, which involves specialized mechanosensitive cells converting mechanical force into electrochemical signals using mechanoreceptors.
One important example is the epithelial enteroendocrine (EEC) cells, specifically enterochromaffin cells, which release the neurotransmitter serotonin when mechanically stimulated. The released serotonin is then transduced (mechanotransduction) and relayed by specialized sensory cells to effector cells, such as smooth muscle cells, to promote GI motility.
While enteroendocrine cells (EECs) respond to mechanical cues in their environment, there is a lack of data on their basic mechanical properties. Understanding these properties, such as rigidity and viscosity, could provide valuable insight into the physiological and pathological changes in these cells. However, studying EECs has been challenging due to their rarity and limited accessibility for in vitro studies. Existing human EEC-immortalized cell lines differ significantly from their wild-type counterparts.
An alternative solution lies in adult stem cell (ASC)-derived organoids, which are three-dimensional structures created from tissue biopsies and composed entirely of primary epithelial cells. These organoids closely mimic the in vivo environment, making them a valuable tool for studying epithelial physiology, stem cell differentiation dynamics, and human disease. As they accurately represent human tissues, these organoids have the potential to advance our understanding of gut cell mechanics.
This study provides a new understanding of the mechanical properties of organoid-derived human enteroendocrine cells (EECs) at the single-cell level. They have conducted the first mechanical analysis of serotonin-producing EECs.
They found that these cells have stiffness values ranging from 60 to 70 pN/μm at stretching velocities ranging from 1 to 20 μm/s. These stiffness values are higher than those observed for red blood cells, and slightly lower than those for primary human monocytes. The Clevers group had created a detailed map of gene expression and secreted proteins in EECs derived from organoids.
In this study, the researchers observed TDO2 expression in duodenal EECs located in the proximal intestine. TDO2 is a key enzyme involved in the breakdown of tryptophan, a precursor of serotonin. Interestingly, when TDO2 is disabled in mice, serotonin levels increase, indicating that TDO2 may regulate local serotonin production in the gut. Given the intricate connection between cellular metabolism and mechanics, they investigated changes in EEC stiffness upon inhibiting TDO2 as a preliminary investigation.
The data presented not only demonstrate the new techniques available for studying the mechanical properties of gut cells but also enhance the understanding of how cell mechanical forces influence their function.
More information: Tom M.J. Evers et al, Mechanics of serotonin-producing human entero-endocrine cells, Mechanobiology in Medicine (2024). DOI: 10.1016/j.mbm.2024.100044