This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Scientists discover how the physics of colon cancer cells contributes to metastasis
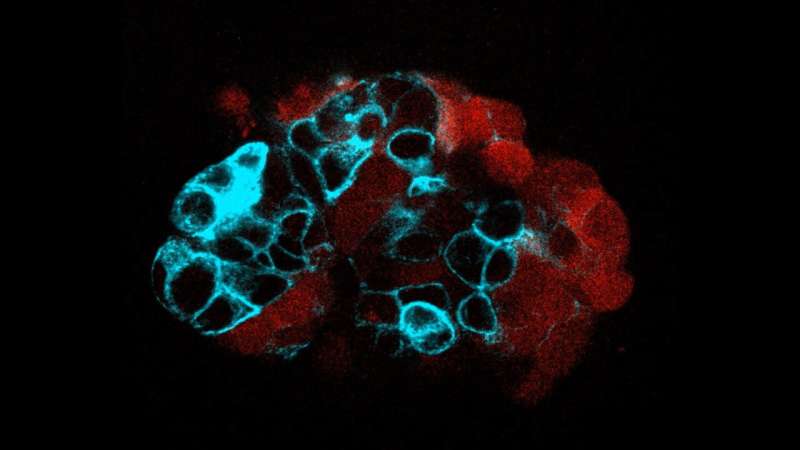
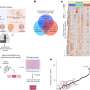
A study led by the Institute for Bioengineering of Catalonia (IBEC) has investigated how the mechanical properties of different types of colorectal cancer cells influence the process of metastasis. The study, which used patient-derived tumor organoids, focused on cancer stem cells expressing the LGR5 protein, a key marker in the signaling required for the renewal and differentiation of intestinal and cancer stem cells. The results showed significant differences in mechanical properties between cells that express LGR5 (LGR5+) and those that do not (LGR5-).
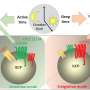
Specifically, the researchers observed that cells that do not express LGR5 are softer, less sticky and move faster, making them better able to move away from the main tumor. In contrast, cells that express LGR5 are more able to adhere to blood vessel walls and create gaps that allow them to invade other tissues, facilitating their growth to new sites in the body, potentially forming metastases.
"This transition between these two cell types that work together is an adaptive mechanism that helps them withstand the physical changes in the microenvironment and facilitates cancer progression," explains Xavier Trepat, ICREA research professor at IBEC.
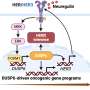
The study, published in the journal Nature Communications, shows that these differences in the mechanical properties of the cells are due to the decrease in ERM proteins (ezrin, radixin and moesin) that occurs in LGR5+ cells.
These proteins link the cell membrane to the internal skeleton of the cell, affecting its stiffness and adhesion. By manipulating these proteins, the research team was able to induce LGR5+ and LGR5- cells to behave similarly, confirming the critical role of ERMs in the observed mechanical differences.
Sefora Conti, IBEC researcher in Trepat's group and first author of the study, explains that they carried out several experiments to test the effect of ERM, "We added a synthetic protein that mimics the function of ERM to LGR5+ cells and observed that they became mechanically indistinguishable from LGR5- cells.
"On the other hand, we reduced the expression of ERM proteins in LGR5- cells and again observed that their mechanical differences from LGR5+ cells were reduced. These results demonstrate the potential of ERM proteins as a therapeutic target to interfere with the metastatic process in colorectal cancer."
In collaboration with Eduard Batlle's group at the Institute for Research in Biomedicine (IRB) in Barcelona, the researchers also analyzed the expression patterns of ERM proteins in individual cells from a cohort of patients and showed that this reduction in ERMs is a common feature of colorectal tumors.
These findings could contribute to the development of new strategies for the treatment and prevention of metastasis in colorectal cancer, which is the third most common cancer worldwide and the second leading cause of cancer-related deaths.
Trepat, who led the research, is also a professor at the University of Barcelona (UB) and a member of the Centre for Biomedical Research Network in Bioengineering, Biomaterials and Nanomedicine (CIBER-BBN). The work was carried out in collaboration with the Centre for Genomic Regulation (CRG) in Barcelona, the Institute for Research in Biomedicine (IRB) in Barcelona, the Max Planck Institute for the Science of Light in Erlangen, Germany, the European Molecular Biology Laboratory (EMBL) in Heidelberg, Germany, the University of Palermo, Italy, and the Príncipe Felipe Research Centre (CIPF) in Valencia.
More information: Sefora Conti et al, Membrane to cortex attachment determines different mechanical phenotypes in LGR5+ and LGR5- colorectal cancer cells, Nature Communications (2024). DOI: 10.1038/s41467-024-47227-2