This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Study shows pre-targeted dual-isotope radionuclide therapy safe and effective in colorectal cancer
Combination of alpha- and beta-radionuclide therapy is feasible, tolerable, and effective for colorectal cancer, according to preclinical research presented at the 2024 Society of Nuclear Medicine and Molecular Imaging Annual meeting. This new approach allows simultaneous delivery of two radionuclides directly to tumor cells while significantly reducing the risk of treatment-related side effects.
Despite the successes of targeted radionuclide therapy, challenges remain; some patients do not respond to the chosen radionuclide therapy, while others experience relapse or develop treatment resistance. Tumor heterogeneity presents another obstacle for targeted radionuclide therapy as differences in tumor make-up can impact effectiveness.
"Combining radionuclides with different radiobiological properties and administering them simultaneously may help overcome some of these issues," said Sara S. Rinne, postdoctoral associate in radiology at Weill Cornell Medicine in New York, New York.
"The use of both alpha- and beta-emitting nuclides could better address tumor heterogeneity due to their varied penetration depths and ability to induce different radiobiological effects, thus creating more complex damage to the tumor and offering a potentially successful treatment approach for a larger patient population."
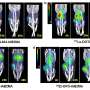
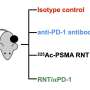
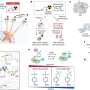
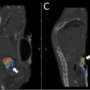
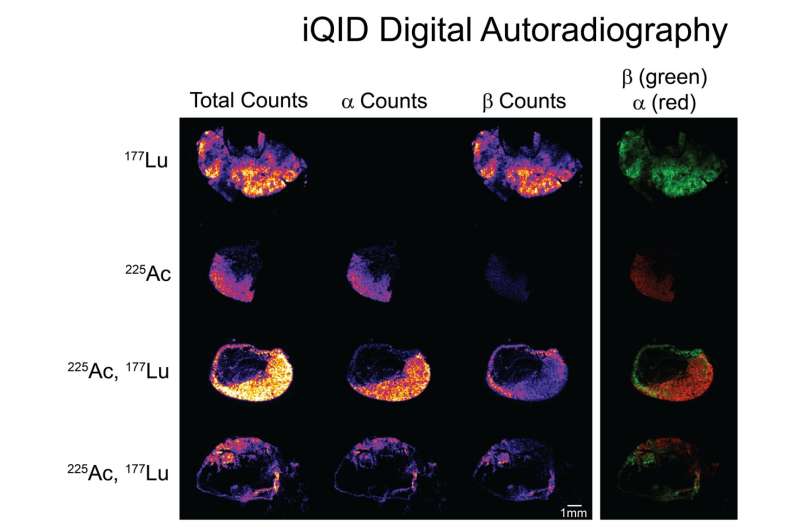
The study aimed to evaluate the feasibility and therapeutic efficacy of simultaneously administered 177Lu/225Ac pretargeted radioimmunotherapy and compare it to separate 177Lu and 225Ac therapies on human colorectal cancer xenografts in mice. Biodistribution and autoradiography experiments were conducted to determine feasibility and dosage. Mice with colorectal cancer xenografts were then treated with mono- or combination therapy and monitored over several months.
Biodistribution and autoradiography experiments confirmed that the combination therapy could bind to colorectal cancer xenografts. Combined 177Lu/225Ac pretargeted radioimmunotherapy was equally as potent as mono-therapy in inducing tumor shrinkage and cures and was well-tolerated in the preclinical model.
"The ability to simultaneously deliver different radioisotopes with complementary radiobiological properties creates new opportunities for improving therapy outcomes and improving patient care," noted Rinne.
"Furthermore, our study's results highlight the great promise and versatility of pretargeted radioimmunotherapy to treat with curative intent. Overall, pre-targeted dual-isotope therapy has the potential to be a versatile approach to help a larger patient population and overcome existing barriers to successful targeted radionuclide therapy."
More information: Rinne et al. 177Lu/225Ac pretargeted dual-isotope therapy for treatment of GPA33(+) colorectal cancer. (2024). Link to Session