This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Researchers publish first computational insights into colonic motility to aid understanding of ulcerative colitis
Ulcerative colitis (UC) is an inflammatory bowel disease (IBD) that causes inflammation and ulcers (sores) in the digestive tract. Ulcerative colitis affects the innermost lining of the large intestine, also called the colon and rectum. At least 40,000 people are living with IBD in Ireland, and over 5 million globally.
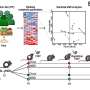
In a new paper recently published in Computers in Biology and Medicine, researchers from CÚRAM at the University of Galway and collaborators at the University of Birmingham present a novel computational model simulating shear stress distribution in the colon with varying mucus thicknesses.
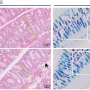
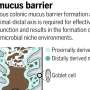
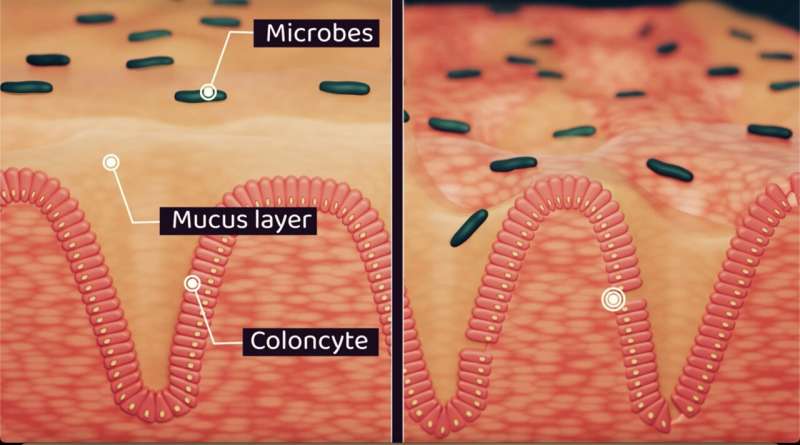
The colon, a vital part of our digestive system, relies on rhythmic contractions to move waste. These movements create mechanical forces on the colon's mucus-lined surface. This mucus acts as a protective barrier, separating the inside of our body from the trillions of microbes in our gut.
The research team's computational model uses real-world data on these movements, mucus behavior and tissue characteristics to simulate the dynamic environment. Their study reveals how the mucus layer acts as a lubricant, significantly increasing fecal velocity and easing waste movement through the colon. This effect is diminished in UC, where the mucus layer is thinner, potentially contributing to constipation.
The model highlights the protective function of mucus, shielding the thin cell layer that performs the colonic processes from the mechanical forces.
When the research team, led by Dr. Yury Rochev, School of Physics, University of Galway, investigated the mechanical distribution in the cell layer, they found that shear stress varies along the various functional zones, suggesting a potential role in regulating cell migration, differentiation, and immune responses. However, when this protection is compromised, such as in the UC, it could contribute to inflammation and tissue damage.
Dr. Rochev said, "Our model demonstrates that mucus acts as a lubricant, significantly increasing fecal velocity and easing waste movement through the colon. This effect is diminished in ulcerative colitis (UC), where the mucus layer thins, potentially contributing to constipation."
The model's revelations do not stop there. It also highlights the protective function of mucus, shielding the delicate cells responsible for essential colonic processes from the mechanical forces generated by bowel movements.
Dr. Rochev added, "When we investigated the mechanical distribution in the cell layer, we found that shear stress varies along the different functional zones, suggesting a potential role in regulating cell migration, differentiation, and immune responses. When this protection is compromised, as in UC, it could contribute to inflammation and tissue damage."
The team is not relying solely on computer simulations. They are developing an experimental model using "organ-on-a-chip" technology to validate their findings.
Ibrahim Erbay, a researcher on the team, said, "We are using intestinal organoids to create a replica of the thin cell layer at the colonic surface. By actively flowing fluid with the organ-on-a-chip platform, we can simulate the mechanical forces similar to those experienced in the colon."
By combining computational modeling with robust experimental validation, the researchers aim to gain a comprehensive understanding of how mechanical forces influence biological events in both health and disease. This holistic approach promises to improve our understanding of gut health and pave the way for new, targeted therapies for inflammatory bowel diseases and other digestive disorders.
Researcher Erbay added, "This research not only enhances our understanding of basic colon function at the cellular level, but also offers a powerful tool for developing new therapeutic approaches. We can now model various drug delivery systems and optimize them, potentially leading to more effective treatments for gut-related conditions."
More information: I.H. Erbay et al, Computational insights into colonic motility: Mechanical role of mucus in homeostasis and inflammation, Computers in Biology and Medicine (2024). DOI: 10.1016/j.compbiomed.2024.108540