This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Scientists develop novel antibody treatment for kidney cancer
Advanced clear cell renal cell carcinoma (ccRCC) is a deadly form of kidney cancer with few treatment options; even with new immunotherapies, only around one in 10 patients ultimately survive.
Antibody therapies called bispecific T cell engagers (BTEs) have emerged as effective treatments for some blood cancers but have been more difficult to develop for solid tumors. While clinically successful, first-generation BTEs suffer a short half-life.
Now, Wistar scientists have built upon BTE technology to develop new and improved recombinant and synthetic DNA versions of therapeutic antibodies that target CA9, called Persistent Multivalent T Cell Engager (CA9-PMTE), that shows promise in pre-clinical models as a potent, long-lasting treatment against ccRCC.
In this study, the researchers also demonstrated that the more potent therapy could be delivered using synthetic DNA, which allows therapeutic production directly in patients. The work is published in the journal Journal for ImmunoTherapy of Cancer.
"The big takeaway is that there may one day be a promising new therapy for kidney cancer that has a mechanism of action that would be compatible for combination with checkpoint inhibitors, which is the current therapy of choice for this type of cancer," said first author Ryan O'Connell, a predoctoral trainee in the Weiner lab at The Wistar Institute's Vaccine & Immunotherapy Center.
"What's more, this improved bispecific antibody is outperforming the traditional bispecific antibodies in our studies, both in efficacy for treating ccRCC and in the approach's ability to last much longer in the body, thus potentially being treatment-sparing."
One reason clear cell renal cell carcinoma is so difficult to treat is because it is a so-called "cold" tumor—one in which cancer cells are unrecognizable by immune cells. This means that killer T-cells—a type of immune cell that seeks out and destroys diseased cells and cancers—are unable to recognize the tumor cells. As a result, immunotherapies that work by enhancing the T cells' killing potency without improving their ability to bind to their targets are less effective against cold tumors.
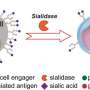
These new forms of bispecific T cell engagers overcome this problem by functioning like "double-sided tape," O'Connell explains. One side of the drug molecule binds to the T-cell, while the other side is engineered to bind to the specific type of tumor cell being treated; these molecules are "bispecific" because each end of the molecule is specific to one of two targets, the T cells and the cancer cells. This empowers the T-cells to attack and kill the cancer—even in cold tumors—by supplementing their ability to bind to the tumor.
But while BTEs are a promising new therapy for many difficult-to-treat cancers, they do have some limitations, including a short half-life (which is how long it takes for the active dose of a drug in one's body to decrease by 50%). Most BTE drugs break down quickly, sometimes within a matter of hours, which means they are only effective for a short time.
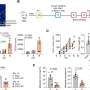
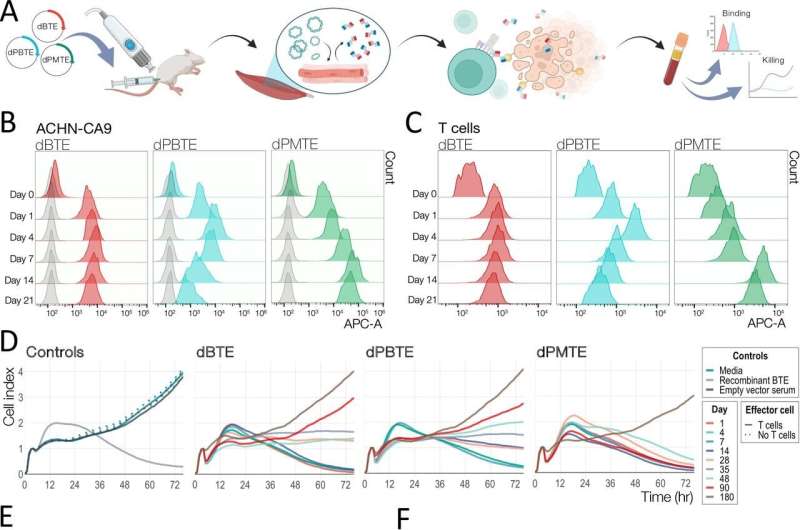
In preclinical models, the team tested the efficacy of novelly designed anti-ccRCC BTE variants developed to enhance the interactions between T cells and the targeted cancer. These were developed to be delivered using synthetic DNA technology—a method of delivery that allows the body to assemble the desired drug design from DNA-based code themselves.
The researchers compared traditional BTEs with a newer format design termed persistent BTEs (PBTEs), which have a longer half-life but use the same targeting system as older BTEs. They found that, while the initial PBTEs did last longer than the traditional BTEs, the new design reduced the overall anticancer potency.
The research team then created a new molecule by taking an existing PBTE and adding additional binding domains to better "see" and bind to the cancer. This novel, alternative design—called a persistent multivalent T cell engager (PMTE)—proved to be highly potent while also maintaining a longer half-life than the traditional BTE design.
Senior author David Weiner, Ph.D., executive vice president of The Wistar Institute and director of the Vaccine & Immunotherapy Center, said the new format represents the potential for an important new tool for enhancing cancer therapy.
"Bispecifics in general are an important technology that offer significant advantages in on-target anticancer potency," he says. "The new PMTEs appear not only more effective at binding to tumor cells and killing the cancer, but they also require a much lower dose and, we have reason to believe, a lower frequency of therapy—which could potentially translate to improved outcomes and a better patient experience at a lower cost."
The researchers are now studying these new PMTEs in combination with other immunotherapies as well as expanding designs to additional difficult-to-treat cancers.
More information: Ryan P O'Connell et al. Format-tuning of in vivo-launched bispecific T cell engager enhances efficacy against renal cell carcinoma, Journal for ImmunoTherapy of Cancer (2024). DOI: 10.1136/jitc-2023-008733. jitc.bmj.com/content/12/6/e008733