This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Suppressing graft-versus-host disease using immunosuppressive iPS cell-derived regulatory T cells
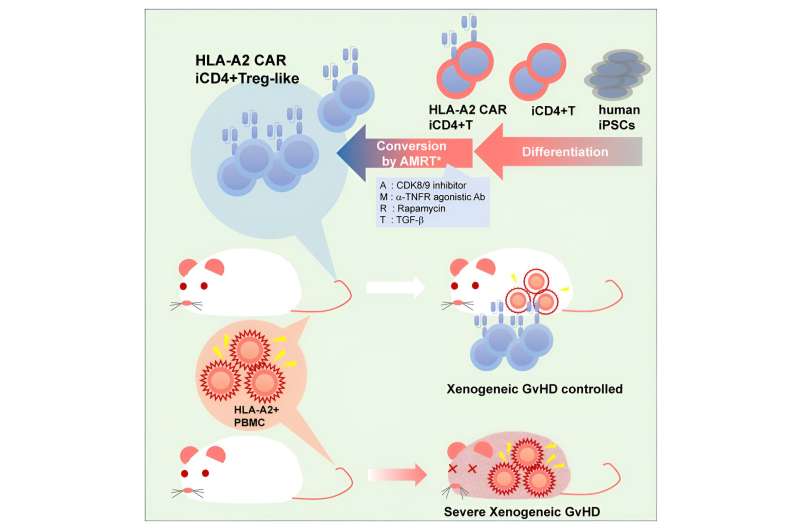
A recent study by a team led by Professor Shin Kaneko successfully induced regulatory T cell (Treg)-like cells from iPS cell-derived conventional helper T cells (Tconvs) and demonstrated their ability to suppress xenogenic graft-versus-host disease (GvHD). The findings are published in the journal Cell Stem Cell.
Autoimmune diseases (e.g., rheumatoid arthritis and myasthenia gravis) and graft-versus-host disease (e.g., from hematopoietic stem cell transplantation) are caused by excessive immune responses.
Immunosuppressive regulatory Tregs can inhibit such undesired immune reactions and are thus seen as a novel therapeutic strategy for treating those conditions. However, challenges remain for expanding Tregs ex vivo to manufacture large quantities of Tregs for clinical use and maintaining their immunosuppressive activity before transplantation.
The team has been working on redifferentiating iPS cells into T cells, but traditional approaches have largely yielded cytotoxic T lymphocytes (CTLs).
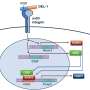
Based on the recently reported artificial thymic organoid (ATO) system capable of differentiating CD4 helper T cells, the researchers hypothesized that it may be possible to convert a subpopulation of such Tconvs into Tregs. Although they successfully differentiated iPS cells into CD4 T cells (iCD4+ T cells) using the ATO system, they also noted that the resulting cells did not express much FOXP3, a master transcription factor for Tregs.
To induce conversion of these iCD4+ T cells into Tregs, the researchers treated them with the following combinations of chemicals and cytokines for 14 days: IL-2 alone, IL-2 + AS2863619 + rapamycin (AR), IL-2 + AS2863619 + rapamycin + TGF-β (ART), IL-2 + AS2863619 + MR2-1 + TGF-β (AMT), and IL-2 + AS2863619 + MR2-1 + rapamycin + TGF- β (AMRT).
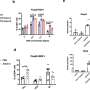
These cocktails were tested for their ability to induce the expression of FOXP3 in iCD4+ T cells, with AMRT treatment showing the highest enhancement in FOXP3 induction. Because FOXP3 is also induced during human T cell activation, the research team also examined another marker, the demethylation of FOXP3 CNS2, a Tregs-specific demethylated region (TSDR), to indicate clearly whether FOXP3+ Tregs were successfully generated.
With this marker, treating iCD4+ T cells with AMRT resulted in similarly high FOXP3 CNS2 TSDR demethylation rates as primary Treg. The research team next performed single-cell RNA sequencing to examine the cellular identities in detail. Aside from FOXP3, they observed that AMRT-induced Tregs from iCD4+ T cells expressed CTLA4, which functions as an immune checkpoint and suppresses immune responses, and other genes known to be expressed in Tregs, such as IL2RA and IKZF2.
Compared to primary and induced Tregs, while the predominant subpopulation of iCD4+ T cells treated with ARMT showed a gene expression signature resembling induced Tregs and characteristic of effector function-related genes, several smaller subpopulations displayed similarities in gene expression profiles to other primary or induced Tregs that may have more specific functions.
These findings collectively reveal that the ARMT cocktail successfully converted iCD4+ T cells to Treg-like cells.
Having successfully generated Treg-like cells from iCD4+ T cells, the researchers tested whether they functionally behave like Tregs.
Using mixed lymphocyte reaction (MLR) assays in which stimulated donor CTLs with primary or induced Tregs or AMRT-treated iCD4+ T cells, they determined that iCD4+ cell-derived Treg-like cells can suppress CTL activation in culture.
In addition, they expanded this analysis to determine whether antigen-specific immunosuppression via a chimeric antigen receptor (CAR) would make AMRT-treated iCD4+ T cells more effective at suppressing CTL proliferation.
Notably, the research team observed that AMRT-treated iCD4+ T cells showed comparable suppressive effects on CTLs regardless of CAR inclusion, thus suggesting that a local and general (non-specific) immunosuppressive effect by the Treg-like cells was sufficient at inhibiting CTLs.
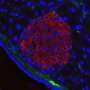
With promising results from these in vitro experiments, the researchers thus extended their study to animal studies to determine the effectiveness of these AMRT-treat iCD4+ T cells at suppressing xenogenic GvHD in vivo.
For this, peripheral blood mononuclear cells (PBMCs) were injected into mice with primary or induced Tregs or AMRT-treated iCD4+ T cells with or without CAR.
Consistent with in vitro observations, Treg-like cells generated by the researchers from iPS cells inhibited weight loss and prolonged survival, similar to primary and induced Tregs. However, a significant effect was not observed in one case, potentially due to the severity of the xenogenic GvHD.
While the research team demonstrated the in vitro and in vivo efficacy of iPS cell-derived Treg-like cells in this study, there is clearly room for further improvements. Nonetheless, their work illustrates the feasibility and potential of using iPS cell-based cell therapy for GvHD and possibly autoimmune diseases.
More information: Hisashi Yano et al, Human iPSC-derived CD4+ Treg-like cells engineered with chimeric antigen receptors control GvHD in a xenograft model, Cell Stem Cell (2024). DOI: 10.1016/j.stem.2024.05.004