This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
reputable news agency
proofread
Study show tirzepatide beneficial for resolution of MASH in patients with MASH, fibrosis
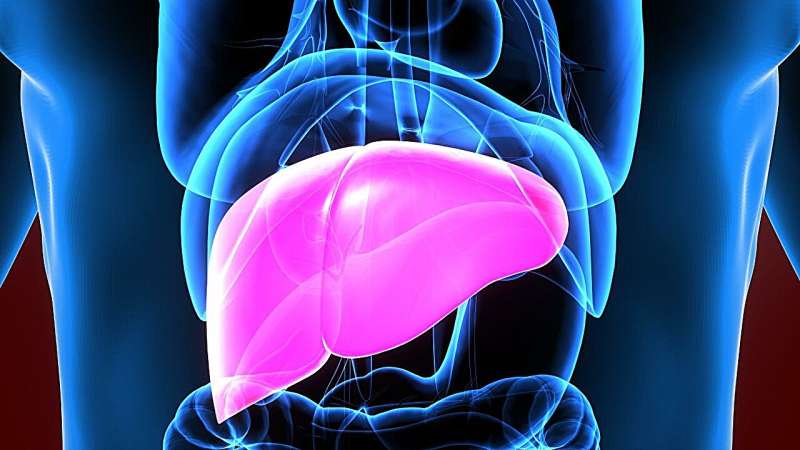
For patients with metabolic dysfunction-associated steatohepatitis (MASH) and moderate-to-severe fibrosis, 52 weeks of tirzepatide is more effective than placebo for resolution of MASH, according to a study published online June 8 in the New England Journal of Medicine to coincide with the annual congress of the European Association for the Study of the Liver, held from June 5 to 8 in Milan.
Rohit Loomba, M.D., from the University of California at San Diego in La Jolla, and colleagues conducted a phase 2, dose-finding, placebo-controlled trial involving participants with biopsy-confirmed MASH and stage F2 or F3 (moderate or severe) fibrosis. Participants were randomly assigned to receive 52 weeks of 5-, 10-, or 15-mg once-weekly subcutaneous tirzepatide or placebo.
Overall, 157 of 190 participants who had undergone randomization had liver-biopsy results at week 52 that could be evaluated, with missing values imputed. The researchers found that the percentage of participants who met the criteria for resolution of MASH without worsening of fibrosis was 10, 44, 56, and 62 in the placebo and 5-, 10-, and 15-mg tirzepatide groups, respectively.
The percentage of patients who had an improvement of at least one fibrosis stage without worsening of MASH was 30 in the placebo group and 55, 51, and 51 in the 5-, 10-, and 15-mg tirzepatide groups, respectively. Gastrointestinal events were the most common adverse events reported in the tirzepatide groups and were mostly mild or moderate in severity.
"Larger and longer trials are needed to further assess the efficacy and safety of tirzepatide for the treatment of MASH with liver fibrosis and to determine whether tirzepatide treatment could reduce the risk of major adverse liver outcomes," the authors write.
Several authors disclosed ties to biopharmaceutical companies, including Eli Lilly, which manufactures tirzepatide and funded the study.
More information: Rohit Loomba et al, Tirzepatide for Metabolic Dysfunction–Associated Steatohepatitis with Liver Fibrosis, New England Journal of Medicine (2024). DOI: 10.1056/NEJMoa2401943
© 2024 HealthDay. All rights reserved.