This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Array pinpoints imprinted genes with potential links to disease
Researchers from North Carolina State University have developed an array that assesses methylation levels of genes located in imprint control regions (ICRs) within the human genome. The array represents a cost-effective, efficient method for exploring potential links between environmental exposures and epigenetic dysregulation during the early developmental origins of diseases and behavioral disorders.
ICRs regulate the expression of imprinted genes—genes where only one parental copy of the gene is active, while the other copy is silenced early in development. Imprinted genes are of special interest to epidemiologists, geneticists, and toxicologists who study the connections between environmental influences and disease because the methylation marks that control their expression are susceptible to environmental influences.
These DNA methylation modifications can be stable throughout the life of the affected individual and may even be passed on to their children. This is of particular interest in epigenetics, which is the study of heritable changes in gene expression in the absence of DNA sequence changes.
"Methylation—whether a gene is 'off' or 'on'—is the easiest thing to look at when you're investigating epigenetic effects," says Cathrine Hoyo, professor of biological sciences at NC State and co-corresponding author of the work. "It serves as a jumping off point for figuring out relationships between the environment and gene expression."
While methylation arrays do exist for investigating gene expression, the ones most commonly used do not include probes specific to ICRs. Instead, scientists interested in imprinted gene regulation must sequence the entire genome of a subject, which is costly, time-consuming and impractical in large population studies.
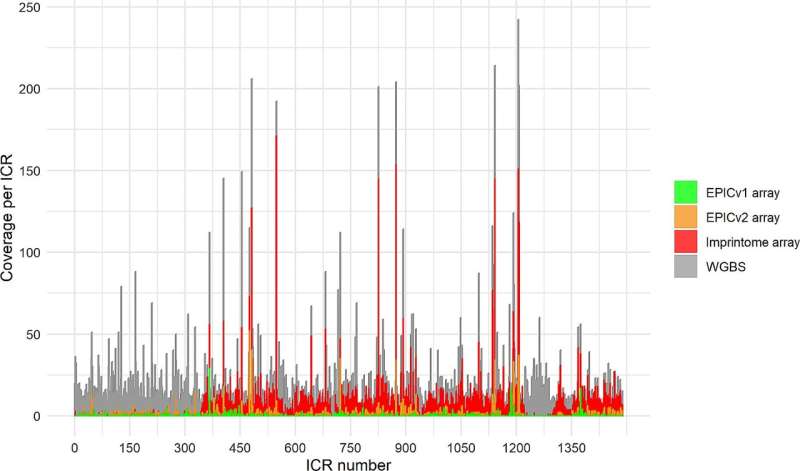
The new array contains 22,000 fluorescent probes that are specific to 1,000 of the known 1,488 ICRs in the human genome. The probes are short DNA sequences that target specific methylation sites within these ICRs, with alternate probes binding the methylated and unmethylated versions. The alternate probes for each target site have different fluorescent signals, so that the relative amount of each probe bound can be measured, and the methylation level for each site determined from the ratio of the specific probes.
As proof of concept, the research team used DNA from a set of Alzheimer's patients to compare ICR methylation data from the array data to the methylation results obtained by whole genome sequencing, and found a significant correlation between the two methods. Most notably for clinical application, the results were complete after one week, as compared to potentially months required for interpreting full genomes.
"In large studies we have to screen the participants," Hoyo says. "If there are 1,000 people in a study, it simply isn't possible to do that many complete genomic sequences in a timely and cost-effective way. It is especially wasteful when you consider that we are only interested in 22,000 sites out of millions in the genome.
"This array does the screening for us—it looks only at the sites of interest and allows us to focus time and energy on full sequencing only when necessary. Essentially it sifts the wheat from the chaff so we can focus only on the ICRs that may be involved in disease."
The work appears in Epigenetics Communications. Other co-authors include first author Natalia Carreras-Gallo, Varun B. Dwaraka, and Tavis L. Mendez from TruDiagnostic; Dereje D. Jima, David A. Skaar, Antonio Planchart and Randy L. Jirtle from NC State University; and Wanding Zhou from the University of Pennsylvania.
More information: Natalia Carreras-Gallo et al, Creation and validation of the first infinium DNA methylation array for the human imprintome, Epigenetics Communications (2024). DOI: 10.1186/s43682-024-00028-6