This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers examine cancer drug guidelines and FDA approvals
Drugs for serious or life-threatening diseases can receive expedited U.S. Food and Drug Administration (FDA) review, allowing patients to receive faster access to promising new treatments. Under the expedited review pathway of accelerated approval, drugs can be FDA-approved based on surrogate markers or proxy measures such as changes in imaging or lab tests that are "reasonably likely" to predict whether patients improve in how they feel, function, or survive.
The FDA has outlined four expedited review pathways for drug approval. In one route, the agency requires drug sponsors of accelerated approval drugs to conduct post-approval trials that are then used by the FDA to determine if a drug should go on to receive traditional FDA approval or be withdrawn from the market. Many accelerated approval drugs are for use in cancer treatment.
Clinicians who prescribe these drugs rely on guidelines from the National Comprehensive Cancer Network (NCCN) for critical information about which drugs to consider for patients and the underlying evidence behind them. These guidelines also directly inform coverage by the Centers for Medicare and Medicaid Services (CMS).
Two recent studies led by Maryam Mooghali, MD, MSc, postdoctoral associate at the Yale Collaboration for Regulatory Rigor, Integrity, and Transparency (CRRIT) and Reshma Ramachandran, MD, MPP, MHS, assistant professor (general internal medicine), and co-director of CRRIT, examine the complicated FDA approval process for cancer drugs and its downstream impacts on patients and clinicians.
NCCN guidelines and drug approval
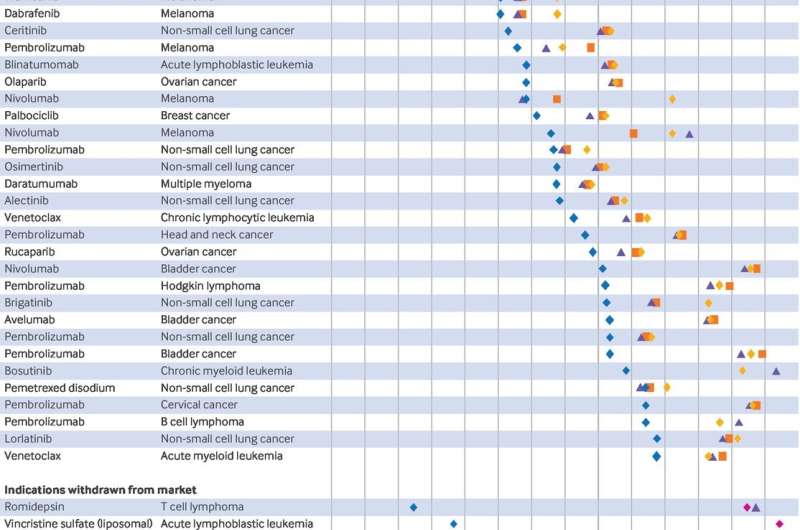
The first study, published in BMJ Medicine, found that in some cases, NCCN guidelines continue to recommend accelerated approval drugs when their post-approval studies failed to confirm clinical benefit or were withdrawn by the FDA.
Additionally, the NCCN guidelines often did not indicate that accelerated approval drugs were based on surrogate markers rather than clinical outcomes or provide information on ongoing post-approval confirmatory trials meant to confirm clinical benefit.
In the article, the authors concluded that NCCN guidelines should be updated in a timely manner in alignment with FDA regulatory decisions. When there is a lack of agreement between NCCN guidelines and FDA decisions or trial results, the authors recommend that guidelines should include an explicit rationale.
Real-time oncology review program and approvals
The second study, published in JAMA Network Open, sought to determine what clinical evidence was available and if post-marketing studies were required after the FDA launched the Real-Time Oncology Review (RTOR) program. The cross-sectional study examined the cancer medications approved between 2018 and 2023 through the use of this additional expedited review pathway.
Through their work, the researchers found that one-fifth of new FDA oncology indication approvals were reviewed under RTOR since its start in 2018 and most of these approvals were based on surrogate markers, rather than clinical outcomes. In addition, a significant proportion did not have required post-approval studies to confirm the clinical benefits.
The results of the studies were striking, even for the research team. "It was surprising that the FDA has approved a significant proportion of cancer drugs through this even faster approval pathway based on surrogate markers and without any requirements to confirm that they improve survival or quality of life for patients," said Mooghali.
She expressed concern over what this means for patient care. "This coupled with a lack of information within clinical practice guidelines about the evidence supporting FDA approval of these cancer drugs is concerning—doctors will continue to assume that these drugs work meaningfully for patients. Even more worrisome is when the evidence is unclear or sometimes, showing the opposite."
Senior author Ramachandran believes significant changes are needed in the FDA approval process for cancer drugs. "Our studies point to the need for FDA to take action to relieve the burden of uncertainty around new drugs for patients and their doctors.
"FDA should require manufacturers to confirm meaningful clinical benefits for all drugs, especially when based on unproven surrogate markers. The agency could also improve its communication with doctors by ensuring that the evidence they review to make regulatory decisions around new drugs is also reflected promptly in clinical practice guidelines.
"These steps would go a long way in instilling trust in the agency."
More information: Maryam Mooghali et al, Characterization of accelerated approval status, trial endpoints and results, and recommendations in guidelines for oncology drug treatments from the National Comprehensive Cancer Network: cross sectional study, BMJ Medicine (2024). DOI: 10.1136/bmjmed-2023-000802
Maryam Mooghali et al, Premarket Evidence and Postmarketing Requirements for Real-Time Oncology Review Indication Approvals, JAMA Network Open (2024). DOI: 10.1001/jamanetworkopen.2024.9233