This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers build first-ever molecular atlas of blood vessel pathways in human brain
An international consortium of researchers led by University Health Network (UHN) in Toronto and University of Zurich have built the first-ever molecular atlas of the human brain vasculature at single-cell resolution, spanning from early development to adulthood and through disease stages such as brain tumors and brain vascular malformations.
The international consortium includes research teams from UHN's Krembil Brain Institute, Donald K. Johnson Eye Institute, Toronto General Hospital Research Institute and Princess Margaret Cancer Center, the University of Toronto's Donnelly Center, Mount Sinai Hospital's Lunenfeld-Tanenbaum Research Institute, University of Zurich, University Hospital Zurich, ETH Zurich, University of Geneva, University Hospital Geneva, as well as collaborators at Weill Cornell Medicine and Memorial Sloan Kettering Cancer Center, in New York.
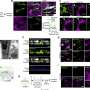
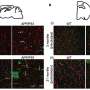
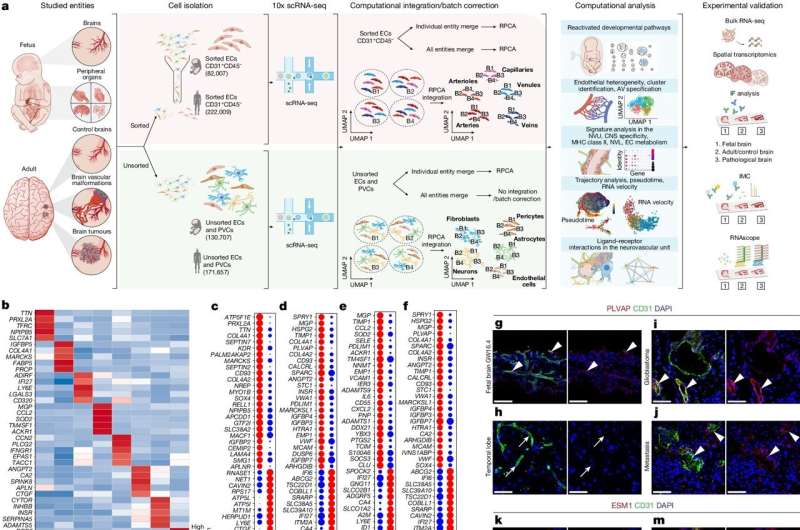
In this study, researchers isolated blood vessels from human early developing brains, adult brains, brain tumors and brain vascular malformations. They found that endothelial cells, which line the blood vessels and regulate interactions between the bloodstream and surrounding tissues, behave differently across various stages of brain development, and may have a more important role than previously understood within the brain's neurovascular signaling networks.
This research is published today in Nature.
"The brain's vasculature, or blood vessel cells, genes, and pathways, is important for the proper functioning of the healthy early developing and adult brain, as well as for a variety of brain diseases, such as brain tumors, stroke and brain vascular malformations," says Dr. Thomas Wälchli, corresponding author of the study and a scientific associate at UHN's Krembil Brain Institute, a consultant neurosurgeon at University College London (UCL)'s Victor Horsley Department of Neurosurgery, and an Associate Professor/Principal Clinical Research Fellow at the UCL Cancer Institute.
"By understanding how these pathways grow and behave during early brain development, how they are silenced in the adult healthy brain, and how they get reactivated in disease, it will provide more insight into the normal functioning of the human brain vasculature and open doors to future therapeutic options," says Wälchli.
"UHN's Krembil Brain Institute is a high-volume neurosurgical center, which provided unparalleled access to a clinically diverse patient population. We were able to perform single-cell RNA sequencing of more than 600,000 isolated endothelial, perivascular and other tissue-derived cells from 117 samples, at an unprecedented resolution, giving us an extraordinary look into the inner workings of the brain's vasculature," says Dr. Ivan Radovanovic, staff neurosurgeon and Senior Scientist at UHN's Krembil Brain Institute, Associate Professor at University of Toronto's Temerty Faculty of Medicine, and co-author of the paper.
"This provides a very large data set that will be an important resource for researchers across the world. By uncovering key differences between healthy and diseased brain vasculature, our work can help identify vulnerabilities of abnormal brain vessels that can be utilized to treat both brain tumors and brain vascular malformations."
Researchers found that the vasculature in an adult healthy brain stops growing almost completely over time, but a brain tumor or a brain vascular malformation can reactivate blood vessel growth in the brain tissue, similar to the blood vessel growth in an early developing brain. This finding has never been described before.
The research team also showed for the first time how the human brain vasculature differs from the vasculature of organs outside the brain, both during early brain development and in adulthood—and when disease arises, the brain vasculature becomes more like that of a peripheral organ.
In disease, the typical features of the human brain vasculature are partially altered. One example is the endothelial cells of the blood-brain barrier, which act as the brain's "filter" and gatekeeper for substances, toxins and drugs. Endothelial cells can also affect interactions with the body's immune system. When disease occurs, these cells help to upregulate immune-specific properties, meaning that endothelial cells can evolve into "antigen-presenting cells," triggering an immune response.
"It will take many years, but if we can identify what is happening in an early developing brain and how those blood vessel networks grow over time, how they develop into arteries, capillaries, and veins, and interact with the immune system, we can better understand the growth patterns of the tumor vasculature," says Dr. Wälchli.
"Both the early developing brain, and brain tumors and brain vascular malformations, feature blood vessel growth and immunosuppression which allow for undisturbed tissue growth. If we can dampen or inhibit the growth of the blood vessels and at the same time boost the immune system, that has a potential application to therapy."
The hope is that if clinicians can one day combine these therapies targeting the vasculature with immunotherapies, they may be able to inhibit vascular growth and prolong patients' survival.
"If we can detect the specific features in the brain vasculature that are shared between early developing brains and brain tumors (but are not present in the healthy normal brain), we could monitor the brain's vasculature for growth patterns and be able to detect and treat disease at an earlier stage, improving patient outcomes," says Dr. Wälchli.
This research aims to build on the momentum in the field of brain vascular biology, over the past several years.
"Our work will benefit scientists across disciplines, from developmental, vascular, computational and tumor biologists to neuroscientists, immunologists and single-cell geneticists," adds Dr. Wälchli. "The possibilities are endless."
More information: Thomas Wälchi, Single-cell atlas of the human brain vasculature across development, adulthood and disease, Nature (2024). DOI: 10.1038/s41586-024-07493-y. www.nature.com/articles/s41586-024-07493-y