This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Study reveals CREB3's role in hepatocellular carcinoma suppression via AKT signaling
New research indicates the inhibitory effects of cAMP responsive element binding protein 3 (CREB3) on hepatocellular carcinoma (HCC) in vitro and in vivo. The paper is published in the journal MedComm.
This study was led by Prof. Bixiang Zhang, Dr. Zhao Huang, Prof. Zeyang Ding, Dr. Huifang Liang (Hepatic Surgery Center, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology). The experimental part was mainly conducted by Dr. Yi He, Dr. Shenqi Han, Ms. Han Li and Dr. Yu Wu.
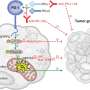
RNA sequencing showed CREB3 regulated AKT signaling to influence HCC progression. Mass spectrometry analysis revealed that CREB3 interacted with an insulin receptor (INSR). Co-immunoprecipitation assay indicated that CREB3 suppressed AKT phosphorylation by inhibiting the interaction of the insulin receptor (INSR) with insulin receptor substrate 1 (IRS1).
In this study, CREB3 was first proved to affect activation of substrates by interacting with tyrosine kinase receptor.
"It is amazing that CREB3 interacts with INSR to inhibit HCC progression. This finding is of great significance," comments Prof. Zhang.
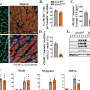
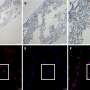
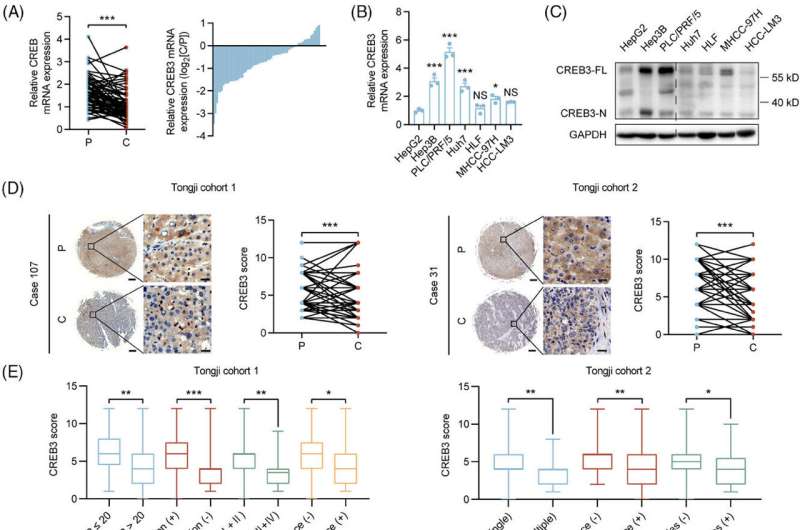
Dr. He and Dr. Han revealed that the in situ protein level of CREB3 was lower in HCCs than in peritumoral tissues in the HCC tissue microarray. HCC tissues of patients with higher alpha fetoprotein (AFP) level (> 20 μg/L), incomplete tumor encapsulation, poorer differentiation grade (III and IV), earlier recurrence (< 2 years), multiple tumor numbers and satellite nodules showed lower CREB3 staining intensity in the microarray.
"Kaplan-Meier analysis showed that high expression of CREB3 predicted longer overall survival and recurrence-free survival, which implied CREB3 might be a tumor suppressor in HCC," Dr. He says.
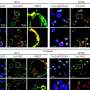
Dr. Li and Dr. Wu also investigated whether CREB3 could act as a transcription factor (TF) in HCC progression. The team verified the intersection of differentially expressed genes in CREB3 mRNA sequencing and genes predicted by the Gene Transcription Regulation Database, and found that RNA-binding motif protein 38 (RBM38) is the target gene.
A dual luciferase reporter and chromatin immunoprecipitation assay identified that CREB3 could act as a TF to transactivate RBM38 expression, leading to suppressed AKT phosphorylation.
The researchers performed rescue experiments to illustrate the relationship of the two functional manners of CREB3 in HCC. The result indicated that knockdown of INSR or overexpression of RBM38 independently attenuates tumor-promoting action caused by CREB3 knockdown. Thus, the team identified that CREB3 suppressed phosphorylation of AKT through both interaction with INSR and transactivation of RBM38 independently.
The team believes the findings in this research will provide a new strategy for HCC therapy.
"AKT signaling is specifically activated in the progenitor subclass of HCC and strongly enhances tumor progression. Our study confirms the dual inhibition mechanism of CREB3 in the HCC focus on AKT signaling. It may play a crucial role in therapy of the progenitor subclass of HCC," Dr. Huang notes.
"In the pathological condition of hyperinsulinemia, insulin mainly stimulates HCC progression through interaction with INSR. For therapy of HCC patients with hyperinsulinemia or insulin resistance, inhibition of INSR signaling appears to be particularly important. It is worth noting that CREB3 suppresses INSR signaling, which probably provides novel insight for therapy of HCC patients with hyperinsulinemia," Dr. He adds.
More information: Yi He et al, CREB3 suppresses hepatocellular carcinoma progression by depressing AKT signaling through competitively binding with insulin receptor and transcriptionally activating RNA‐binding motif protein 38, MedComm (2024). DOI: 10.1002/mco2.633