This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
reputable news agency
proofread
Tisotumab vedotin found efficacious for recurrent cervical cancer
Second- or third-line treatment with tisotumab vedotin is efficacious for patients with recurrent cervical cancer, according to a study published in the New England Journal of Medicine.
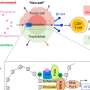
Ignace Vergote, M.D., Ph.D., from Universitaire Ziekenhuizen Leuven in Belgium, and colleagues conducted a phase 3, multinational, open-label trial of tisotumab vedotin as second- or third-line therapy in 502 patients with recurrent or metastatic cervical cancer. Participants were randomly assigned to receive tisotumab vedotin monotherapy (2.0 mg per kilogram of body weight every three weeks) or the investigator's choice of chemotherapy (253 and 249 patients, respectively).
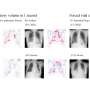
The researchers found that median overall survival was significantly longer in the tisotumab vedotin group versus chemotherapy group, resulting in a lower risk for death (11.5 versus 9.5 months; hazard ratio, 0.70). Median progression-free survival was 4.2 versus 2.9 months for tisotumab vedotin versus chemotherapy (hazard ratio, 0.67). The confirmed objective response rate was 17.8 and 5.2% in the tisotumab vedotin and chemotherapy groups, respectively (odds ratio, 4.0).
At least one adverse event that occurred during the treatment period was seen in 98.4 and 99.2% of patients in the tisotumab vedotin and chemotherapy groups, respectively; grade 3 or greater events occurred in 52.0 and 62.3%, respectively. Due to toxic effects, 14.8% of patients stopped tisotumab vedotin treatment.
"These data suggest that tisotumab vedotin may be a preferred second-line or third-line treatment option over chemotherapy for patients with recurrent cervical cancer," the authors write.
The study was funded by Genmab and Seagen, which was acquired by Pfizer in December 2023; tisotumab vedotin was codeveloped by Genmab and Pfizer.
More information: Ignace Vergote et al, Tisotumab Vedotin as Second- or Third-Line Therapy for Recurrent Cervical Cancer, New England Journal of Medicine (2024). DOI: 10.1056/NEJMoa2313811
© 2024 HealthDay. All rights reserved.