This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Epigenetic change to DNA associated with cancer risk in 'multi-omics' study

A research team co-led by investigators at Vanderbilt University Medical Center and the University of Virginia has identified associations between DNA methylation and cancer risk. DNA methylation is an epigenetic change—the addition of "methyl groups" to DNA—that can affect gene expression without changing the DNA sequence. Most DNA methylation occurs on CpG sites in the genome.
The new study, published in the journal Nature Communications, identifies 4,248 CpG sites associated with the risk of seven different types of cancer: breast, colorectal, renal cell, lung, ovarian, prostate and testicular germ cell cancers.
"The findings substantially advance our understanding of the interplay between genetics, epigenetics and gene expression in cancer etiology," said Qiuyin Cai, MD, Ph.D., co-corresponding author of the new study with Jirong Long, Ph.D., and Yaohua Yang, Ph.D. Cai and Long are both professors of Medicine in the Division of Epidemiology at VUMC; Yang is assistant professor of Public Health Sciences at the University of Virginia.
Although genome-wide association studies (GWAS) have identified more than 1,000 common genetic variants (changes in DNA sequence) associated with cancer risk, many of these variants are in regions of the genome that do not code for proteins. This poses a challenge for identifying the target genes of these genetic variants and exploring how they impact cell function and contribute to cancer development.
Cai, Long and their colleagues previously identified DNA methylation levels in blood samples associated with cancer risk. They now extend their studies to cancer-relevant tissues.
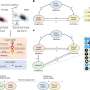
Using DNA methylation data in normal tissues and paired genetic data from cancer-free donors from the Genotype-Tissue Expression consortium, they developed genetic models to predict DNA methylation levels at CpG sites across the genome for seven tissues.
The investigators then applied their prediction models to the corresponding GWAS data for cancers of the seven tissue types to determine associations between genetically predicted DNA methylation levels and cancer risk.
Of the 4,248 CpG sites associated with cancer risk, 95% were specific to a particular cancer type.
For the cancer-associated CpG sites, the researchers conducted integrative "multi-omics" analyses of DNA methylomic, transcriptomic, genomic and cancer GWAS data to explore whether the DNA methylation levels affect cancer risk by modulating the expression of nearby genes.
These studies revealed DNA methylation levels at 309 distinct CpG sites might influence cancer risk by regulating the expression of 205 unique genes.
"Our findings emphasize the effectiveness of multi-omics integration and enhance our understanding of the critical role of genetics and epigenetics in cancer development," Long said.
More information: Yaohua Yang et al, Integrating muti-omics data to identify tissue-specific DNA methylation biomarkers for cancer risk, Nature Communications (2024). DOI: 10.1038/s41467-024-50404-y