This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
reputable news agency
proofread
FDA approves first nasal spray to curb anaphylaxis, an alternative to injections
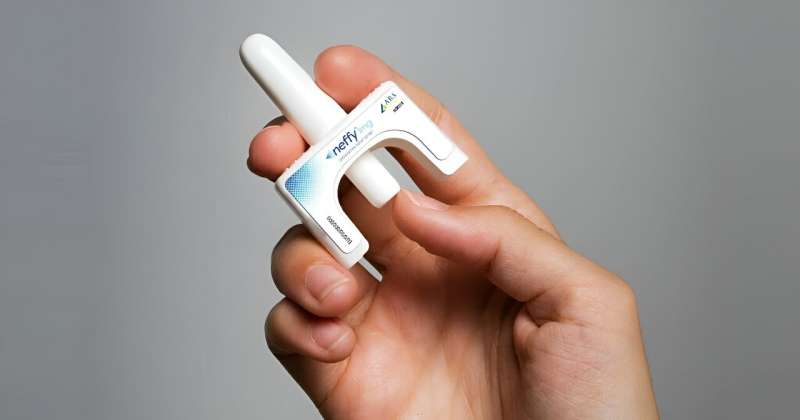
Folks nervous about administering a rescue shot for anaphylaxis finally have a new alternative in a nasal spray.
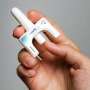
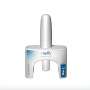
The U.S. Food and Drug Administration on Friday announced that it has approved neffy, the first non-injected treatment for life-threatening allergic reactions.
The epinephrine nasal spray is for use by adults and children who weigh more than 66 pounds, the agency said.
"Anaphylaxis is life-threatening and some people, particularly children, may delay or avoid treatment due to fear of injections," Dr. Kelly Stone, associate director of the Division of Pulmonology, Allergy and Critical Care in the FDA's Center for Drug Evaluation and Research, said in an agency statement. "The availability of epinephrine nasal spray may reduce barriers to rapid treatment of anaphylaxis. As a result, neffy provides an important treatment option and addresses an unmet need."
Folks with allergies can experience a sudden, frightening reaction to allergens—often certain foods, medications or insect stings.
Until now, epinephrine has been the only rescue medication when such incidents occur, and it's only been administered via a needle.
Neffy, made by ARS Pharmaceuticals, is delivered as a spray spritzed into one nostril, the FDA said. If the first dose doesn't ease symptoms, the agency urges giving a second dose (from a new dispenser) into the same nostril. Monitor patients closely after epinephrine is used, in case further treatment, including emergency medical help, is needed.
"Neffy's approval is based on four studies in 175 healthy adults, without anaphylaxis, that measured the epinephrine concentrations in the blood following administration of neffy or approved epinephrine injection products," the FDA said. "Results from these studies showed comparable epinephrine blood concentrations between neffy and approved epinephrine injection products."
The studies also showed that neffy was effective in triggering the kinds of increases in blood pressure and heart rate that are crucial to treating anaphylaxis.
Neffy can come with side effects. These may include: throat irritation, tingling nose, headache, nasal discomfort, feeling jittery, tingling sensation, fatigue, tremor, runny nose, itchiness inside the nose (nasal pruritus), sneezing, abdominal pain, gum pain, numbness in the mouth, nasal congestion, dizziness, nausea and vomiting.
"Neffy comes with a warning that certain nasal conditions, such as nasal polyps or a history of nasal surgery, may affect absorption of neffy, and patients with these conditions should consult with a health care professional to consider use of an injectable epinephrine product," the FDA added. "Neffy also comes with warnings and precautions about use of epinephrine by people with certain coexisting conditions and allergic reactions associated with sulfite."
The new approval comes after an expert advisory panel supported neffy's use back in May of 2023. In September, the FDA held back on approval, asking for further study.
More information: Learn the signs of anaphylaxis at the Cleveland Clinic.
SOURCE: U.S. Food and Drug Administration, announcement, Aug. 9, 2024
Copyright © 2024 HealthDay. All rights reserved.