This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Novel method for treating cerebral ischemic stroke modulates molecular transportation in brain extracellular space
Ischemic stroke is the second leading cause of death globally and the primary cause of death in China. Over the past two decades, Prof. Hongbin Han's team has been committed to advancing diagnosis and treatment of major brain disorders by investigating the brain extracellular space (ECS). Brain ECS occupies 15%–20% of the total brain volume, significantly more than the 3%–5% occupied by cerebral blood vessels, which have so far received much attention.
The paper is published in the journal Science China Life Sciences and was led by Dr. Han at Peking University.
Brain ECS is crucial for brain functional activities such as sleep, memory, and sensory formation. It not only plays a vital role in the onset and progress of brain disorders but also provides the necessary pathway for drugs to enter and reach targets to exert their effects. The development of novel neuroprotective drugs for ischemic stroke has been greatly hindered by the blood-brain barrier (BBB). Fortunately, administrating drugs via ECS can bypass the BBB and allows direct infusion into the brain parenchyma, paving a new way for therapeutics.
A decade ago, the team reported a drug delivery method via ECS (simple diffusion delivery, SDD), which was also published in Science China Life Sciences. This technique overcame significant challenges associated with pressure-based drug delivery methods (convection enhanced delivery, CED), including drug reflux and precise quantitative analysis.
By using SDD, only 1/800 of the conventional citicoline dose is administrated to achieve a six times greater neuroprotective effect on the ischemic region in the tMCAO model. This was the first time to demonstrate the feasibility and efficiency of drug delivery via ECS for treating cerebral ischemic stroke. However, the invasive drug delivery procedure retards the translation from bench to clinical application.
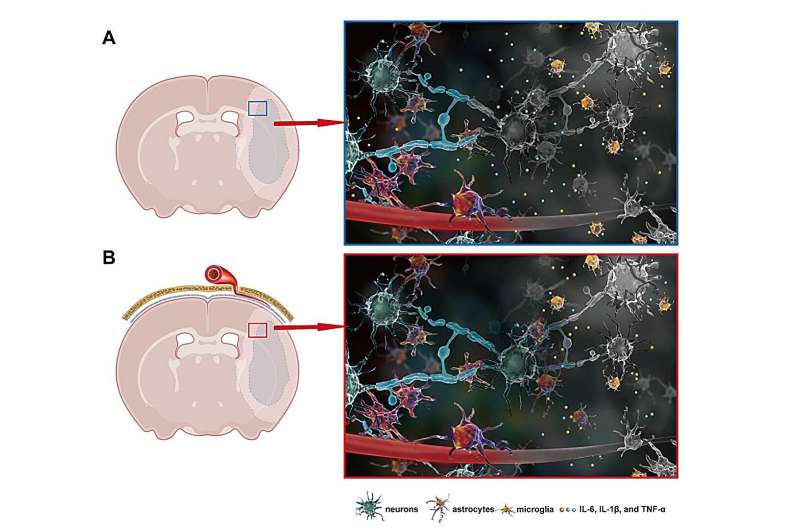
After 13 years of effort, the team recently developed a safer, minimally invasive method, named as modified epidural artery implantation (m-EAI). The enhancing effect of EAI on interstitial fluid (ISF) drainage was verified and assessed using tracer-based MRI. In the current study, the effect was reconfirmed with m-EAI. This modification allows control of ISF drainage acceleration at different time points following an ischemic attack.
The novel method does not require aggressive epidural penetration, but provides the same neuroprotective effect as the invasive drug delivery method. The neuroprotection results from alleviated inflammatory response following the ISF drainage acceleration. The accumulation of pro-inflammatory cytokines in ECS is significantly decreased and the overactivation of microglia and astrocytes is relieved with m-EAl.
In summary, this study proposed a novel method to treat cerebral ischemic stroke by modulating the molecular transportation in the brain extracellular space. Notably, the neuroprotective effect is obtained without any assistance of neuroprotective drugs.
Further study is needed to verify the combined effects of m-EAI and neuroprotective drugs on ischemic stroke. In addition, this study consolidated the hypothesis that inflammatory response exacerbates ischemic damage.
More information: Jingge Lian et al, A novel neuroprotective method against ischemic stroke by accelerating the drainage of brain interstitial fluid, Science China Life Sciences (2024). DOI: 10.1007/s11427-024-2592-4