This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Optimizing electrical stimulation therapies with machine learning
Like a pacemaker for the heart, nerve stimulation devices are implanted to send pulses of electricity to evoke activity in nerves throughout the body. These electrical stimulation devices have been used to treat and control many disorders, including heart disease, epilepsy, depression, and rheumatoid arthritis.
But there are numerous variables that affect exactly how a nerve responds to stimulation, making the development and use of nerve stimulation therapies difficult and complex.
Neural engineers at Duke University have designed a computer model that makes it significantly easier to simulate nerve responses to electrical stimulation. The model is able to simulate the action of more than 50,000 nerve fibers in the time it takes the current industry standard to simulate one. The researchers say this new tool will assist the design of more effective and targeted neuromodulation therapies.
The research appears in the journal Nature Communications and the new tool is freely available.
"There are a lot of possible adjustments that need to be considered to optimize these devices for effective clinical treatment, whether it's altering the amplitude, duration, shape, or frequency of the pulse, or changing the placement of the electrodes," said Warren Grill, the Edmund T. Pratt, Jr. School Distinguished Professor of Biomedical Engineering at Duke.
"The neural responses are affected by the anatomy and characteristics of the nerves themselves. You have a lot of options where you can change the stimulation settings, and it's challenging to know which changes will make the biggest improvement."
Engineers have long relied on a platform called "NEURON" to model how nerve fibers respond to electrical stimulation. The "MRG" model of a nerve fiber is implemented in NEURON and has been used extensively in academic research and industry.
While the MRG model is very accurate, the computing power needed to simulate neural responses limits its speed, creating a bottleneck that prevents MRG's use in real-time modeling and slows research to improve existing therapies.
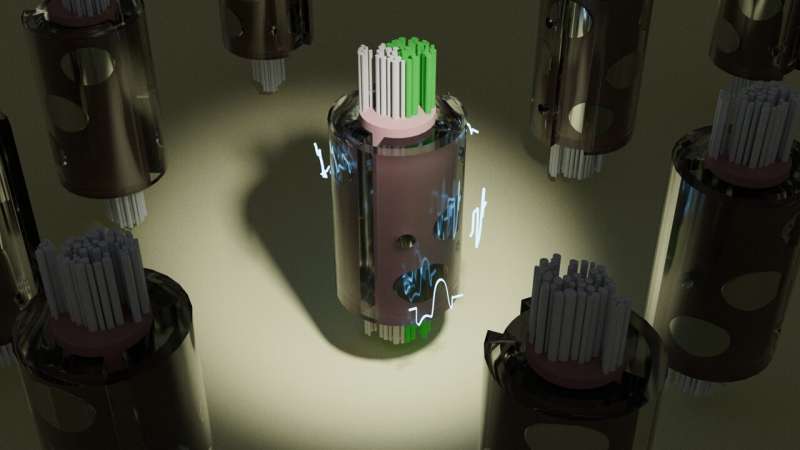
To overcome this long-standing roadblock, Grill, Minhaj Hussain, a Ph.D. student in the Grill lab, and Nicole "Nikki" Pelot, the research director of the lab, developed S-MF (pronounced "smurf"), an alternative to the MRG nerve fiber model. Simulation of a population of S-MF nerve fiber models runs thousands of times faster than a population of MRG nerve fiber models, without sacrificing accuracy or detail.
Unlike NEURON and the MRG model, which run on CPUs (central processing units), S-MF runs on GPUs (graphics processing units), a kind of computer chip that can run thousands of computations in parallel.
"If we model a single fiber, S-MF is not much faster than NEURON," said Hussain. "But the tremendous leap forward lies in the fact that S-MF takes the same amount of time to simulate several thousand nerve fibers as it takes to simulate just one MRG nerve fiber. The human vagus nerve alone contains 100,000 nerve fibers, so this new efficiency is incredibly helpful."
The vagus nerve is a key target for stimulation therapies, as it connects the brainstem to most organs in the torso, including the heart, lungs, pancreas, stomach, and liver. Effective stimulation has been shown to safely treat conditions including drug-resistant epilepsy, depression, and heart failure. However, stimulation of off-target fibers in the nerve can cause side effects.
The team simplified how the anatomy of the nerve fibers is represented in their models: the MRG model represents different micron-level anatomical features along the length of a neuron, while S-MF focuses on the key features that initiate and propagate neural activity. The team used machine learning approaches to define the electrical parameters of S-MF to ensure accuracy comparable to the MRG model.
"Unlike other studies that used alternative approaches to speed up these simulations, S-MF is accurate across a wide range of neural anatomies and stimulation parameters," Nikki Pelot said. "S-MF also retains a lot of detail that other simplifications neglected, which provides important information for designing better therapies."
The team used S-MF to test different stimulation scenarios on thousands of different nerve fibers at once and quickly identify the best conditions for optimal nerve stimulation. The GPU-based design of S-MF enabled the team to use machine learning optimization techniques, which are faster than optimization techniques available for NEURON-based models.
To demonstrate the power of S-MF and its machine learning optimization, the team predicted stimulation parameters that would initiate neural activity only in half of the vagus nerve, while leaving fibers inactive in the other half.
The team's platform quickly and correctly predicted the stimulation levels and patterns that triggered the desired response in models of both human and pig vagus nerves, activating the target nerve fibers while avoiding the off-target nerve fibers.
Although S-MF was trained to mimic the MRG model of myelinated fibers, an important target for neuromodulation therapies, the team also demonstrated that their platform could easily be adapted to simulate other types of nerve fibers.
They are exploring how their approach can be expanded to other neuromodulation techniques including transcranial magnetic stimulation of the brain, which would require modeling a more complex neural anatomy and multiple types of neurons in the brain.
"Neural engineering benefits as a field when we have access to models that are scalable, efficient, and anatomically realistic," said Hussain. "Our hope is that, as we continue to use this platform, it will tell us more about the design decisions we should be making with our stimulation therapies so that we can achieve the best outcomes possible."
More information: Minhaj A. Hussain et al, Highly efficient modeling and optimization of neural fiber responses to electrical stimulation, Nature Communications (2024). DOI: 10.1038/s41467-024-51709-8